In Patients With Colorectal Liver Metastases, Can We Still Rely on Number to Define Treatment and Outcome?
The surgical strategies of “classic, reversed, or combined” resection of colorectal cancer and colorectal liver metastases have to be tailored to a specific patient, and all three strategies have a role in the treatment of stage IV colorectal cancer today.
Figure 1: Number-Independent Survival of 473 Patients With Colorectal Liver Metastasis
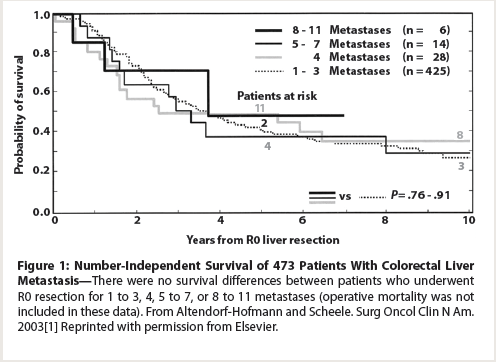
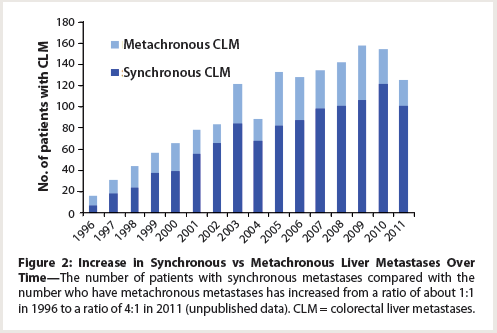
Figure 2: Increase in Synchronous vs Metachronous Liver Metastases Over Time
We congratulate Dr. Weiser and colleagues for an excellent and concise review of the approaches available for colorectal cancer patients with a limited number of metastases, defined as “oligometastatic disease.”[1] This commentary provides us with the opportunity to revisit the concept of “oligometastatic disease” that was developed in the era predating the use of effective chemotherapy, when surgery predominantly defined patient outcomes. At that time, a small number of metastases was considered a sign of favorable biology. Today up to 25 % of patients with metastatic colorectal cancer are candidates for resection of colorectal liver metastases (CLM). Patients with CLM encompass a broad spectrum of clinical presentations, and new concepts of prognostication are emerging.
To achieve optimal outcomes, patients with CLM should undergo a planned sequence of therapeutic interventions that include perioperative chemotherapy and surgery. The surgeon and the medical oncologist are pivotal in keeping the patient on a therapeutic track that will optimize the benefits derived from chemotherapy and surgery. In this commentary, we provide approaches and perspectives that represent alternatives to the important concepts discussed by Weiser et al. We have organized our remarks around five key questions raised by the authors.
1. Should the number of lesions still be included in the criteria used to select patients for surgical resection?
In 2003, Altendorf-Hofmann et al demonstrated no survival differences between patients who underwent R0 resection for 1 to 3, 4, 5 to 7, or 8 to 11 metastases (Figure 1).[2] In light of these data, we think that response to modern chemotherapy may help determine the biology of the disease better than the number of lesions. In recent studies, pathologic response to chemotherapy predicted outcome and outperformed the traditional predictors of outcome, including the number of metastases.[3]These findings may explain why the resection of multiple CLM was associated with more favorable long-term outcomes in previous studies. Because the number of lesions does not necessarily correlate with the biology of the disease, we use high-quality CT scans to assess the radiologic response to chemotherapy-which correlates with pathologic response.[4] The specific radiologic criteria we use go beyond the Response Evaluation Criteria In Solid Tumors and include morphologic changes in the CLM (eg, homogeneous loss of enhancement with well-defined margins) as a size-independent predictor of outcome.[5] In addition, we have shown that RAS mutations (KRAS and NRAS) predict poorer overall and disease-free survival, as well as a pattern of early lung recurrence in patients undergoing resection for CLM.[6,7] On multivariate analysis, RAS mutational status outperformed traditional predictors of outcome, such as the number and size of CLM. Because RAS mutational status is highly concordant (> 90%) between the primary tumor and the CLM, this prognostic information can be obtained early in the evaluation of the disease via pretreatment biopsy or from the resected primary tumor.
2. Does a small number of lesions define the extent of resection and the approach?
One or two lesions may call for limited liver surgery in some patients, but if unfavorably located (eg, involvement of two hepatic veins), these few lesions may necessitate extensive resection. This could have a direct impact on decisions regarding the type and sequence of surgical procedures or the use of perioperative therapy. In patients with unfavorably located lesions, preoperative portal vein embolization may be useful prior to surgery and should be integrated into the multidisciplinary treatment scheme. Portal vein embolization will increase the size of the anticipated liver remnant and reduce the risk of complications associated with extensive resection.[8] Nonetheless, two unfavorably located CLM may preclude combined resection of the primary tumor and metastases because of increased surgical risk.
3. Should CLM be resected at the time of primary colorectal cancer resection (“combined approach”), or should the resection of primary and metastatic lesions be staged?
The two options for staged surgery are the “classic approach,” which is resection of the primary tumor first, and the “reverse approach,” which is resection of the CLM first. Specific factors that have been shown to increase the rate of postoperative complications in combined procedures are the presence of a diverting stoma, rectal location of the primary tumor, duration of the surgery, intraoperative blood loss, and the need for transfusion.[9] The fact that the combined approach could increase the surgical risk for some patients is supported by a large multi-institutional analysis that demonstrated a more than threefold increase in morbidity and mortality for simultaneous colorectal and liver resection compared with hepatectomy alone.[10] Such complications will have a negative impact on the well-defined sequence of chemotherapy and surgery for patients with stage IV disease. The factor that has the greatest impact on long-term survival is the presence of CLM. This needs to be considered in the timing of chemotherapy and surgery for the primary tumor and for CLM. We plan surgery with the goal of removing all liver disease first. This sometimes requires two or three surgical procedures and may include portal vein embolization.[11] In our practice, contrary to the authors’ experience, resecting CLM first is not a “select circumstance” in patients undergoing liver surgery, even in the “era of laparoscopic surgery.”[12] In addition, we recently showed that the morbidity and mortality of proctectomies performed after this “reversed strategy” are similar to the morbidity and mortality of rectal resection performed at the initial surgery.[13]
In patients who have symptomatic primary tumors-who present with bleeding, obstruction, impending perforation, or poorly controlled malignant fistulae-the classic approach of resecting the colon first is still valid. In patients with a favorable primary colorectal tumor and metastatic disease that requires limited liver resection with favorable operative risk, a combined approach is the best choice. Therefore, we think that all permutations of “classic, reversed, or combined” approaches for colorectal/liver resection are valid; the choice should be dictated by the specific patient situation.[12]
4. What is the role of chemotherapy in patients who require resection for CLM?
When discussing the role of chemotherapy in the treatment of CLM today, it is important to include targeted agents. Although no randomized controlled trial to date has specifically looked at the role of VEGF receptor inhibition in the neoadjuvant setting, we recently showed that radiographic response to VEGF inhibition is associated with improved overall and disease-free survival.[5] Synthesizing the data from the European Organisation for Research and Treatment of Cancer (EORTC) 40983 study and our data, we recommend a limited course of preoperative modern chemotherapy (eg, 4 to 6 cycles) along with targeted therapy (eg, 3 to 5 cycles), except in patients who have already received modern adjuvant chemotherapy for the primary tumor within 1 year. This allows us to prevent some patients from undergoing unnecessary surgery or, conversely, to select surgery for patients with borderline resectable disease. In our experience with patients who have undergone resection for synchronous vs metachronous CLM, the number of patients with synchronous metastases compared with the number who have metachronous metastases has increased from a ratio of about 1:1 in 1996 to a ratio of 4:1 in 2011 (Figure 2). Synchronous disease portends a poorer prognosis, and because its incidence is increasing, we favor the use of the most effective preoperative chemotherapy.
5. What is the role of pelvic radiotherapy in the setting of locally advanced rectal cancer and metastatic disease?
Radiotherapy, especially when delivered preoperatively, has proven to be more effective than surgery alone at providing improved locoregional control for locally advanced rectal cancer.[14,15] However, the addition of radiotherapy might not provide significant gains in overall survival benefit in rectal cancer patients with systemic disease, including those with CLM. In a study of 141 patients with stage IV rectal cancer, more than half the patients with local recurrence in the pelvis also had recurrent systemic metastases.[16] Another study of patients with stage IV rectal cancer who were treated with postoperative concurrent chemotherapy and radiotherapy vs chemotherapy alone demonstrated no difference in overall survival (2 years), local recurrence-free survival, or disease-free survival.[17] Thus, the overall oncologic outcomes in this patient cohort are largely determined by metastatic disease rather than by local recurrence. Additionally, the response of the primary rectal cancer to systemic chemotherapy alone is highly variable, with observed histologic response rates ranging from 30% to more than 90% and observed complete pathologic response rates of 6% to 35%, indicating that pelvic irradiation may indeed provide added local benefit in a select subset of patients.[18,19] Therefore, we concur with the authors that the role of pelvic irradiation in patients with metastatic and locally advanced rectal cancer is not uniform, and patient selection must balance the potential benefit of optimal local control against the potential harm in the time away from effective systemic chemotherapy.
In conclusion, the surgical strategies of “classic, reversed, or combined” resection of colorectal cancer and CLM have to be tailored to a specific patient, and all three strategies have a role in the treatment of stage IV colorectal cancer today. The question of how to prognosticate patients with stage IV colorectal disease at diagnosis that is addressed by Weiser et al is a very timely one, given that the proportion of colorectal cancer patients with synchronous CLM is increasing. Since the number of metastases might not help the clinician to accurately prognosticate these patients or define the surgical strategy, we recommend focusing on surgical considerations such as lesion location and the response of the tumor to therapy as surrogate markers of tumor biology. We believe clinical surrogate parameters are a function of the mutational profile of the tumor, which actually determines its biology, and that these surrogate parameters should ultimately be replaced by mutational testing that is precisely correlated to outcomes and patterns of disease.
Acknowledgement:The research discussed in this commentary was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672.
Disclosures:
Dr. Vauthey has received a research grant from, and serves as a speaker for, Roche. Drs. Conrad and You have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Weiser MR, Jarnagin WR, Saltz LS. Colorectal cancer patients with oligometastatic liver disease: What is the optimal approach? Oncology (Williston Park). 2013;27:1074-8.
2. Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am. 2003;12:165-92.
3. Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344-51.
4. Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338-44.
5. Shindoh J, Loyer EM, Kopetz S, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30:4566-72.
6. Kopetz S, Vauthey JN. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. Aug 2013. [Epub ahead of print]
7. Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258:619-27.
8. Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-8.
9. Hillingso JG, Wille-Jorgensen P. Staged or simultaneous resection of synchronous liver metastases from colorectal cancer-a systematic review. Colorectal Dis. 2009;11:3-10.
10. Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481-91.
11. Brouquet A, Abdalla EK, Kopetz S, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083-90.
12. Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934-41.
13. Tzeng CW, Aloia TA, Vauthey JN, et al. Morbidity of staged proctectomy after hepatectomy for colorectal cancer: a matched case-control analysis. Ann Surg Oncol. 2013;20:482-90.
14. Cedermark B. Randomized study on preoperative radiotherapy in rectal carcinoma. Ann Surg Oncol. 1996;3:423-30.
15. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-40.
16. Assumpcao L, Choti MA, Gleisner AL, et al. Patterns of recurrence following liver resection for colorectal metastases: effect of primary rectal tumor site. Arch Surg. 2008;143:743-9; discussion 49-50.
17. Chang CY, Kim HC, Park YS, et al. The effect of postoperative pelvic irradiation after complete resection of metastatic rectal cancer. J Surg Oncol. 2012;105:244-8.
18. Chang G, Maru D, Agarwal A, et al. Histopathologic responses in primary tumors of patients with metastatic colorectal carcinoma after neoadjuvant systemic chemotherapy alone. ESMO Congress Abstracts. 2010;21:P-0032.
19. Cercek A, Weiser MR, Goodman KA, et al. Complete pathologic response in the primary of rectal or colon cancer treated with FOLFOX without radiation. ASCO Meeting Abstracts. 2010;28:3649.