Sequencing of Agents for Castration-Resistant Prostate Cancer
In this review we will outline an approach to sequencing new therapies for metastatic castration-resistant prostate cancer (CRPC), with particular attention paid to the biology of CRPC.
Figure 1: Sites of Androgen Production and Androgen-Blocking Therapies
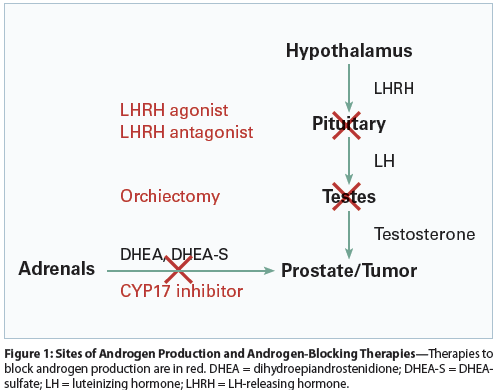
Figure 2: Intracellular Androgen Signaling and Mechanisms of Blockade
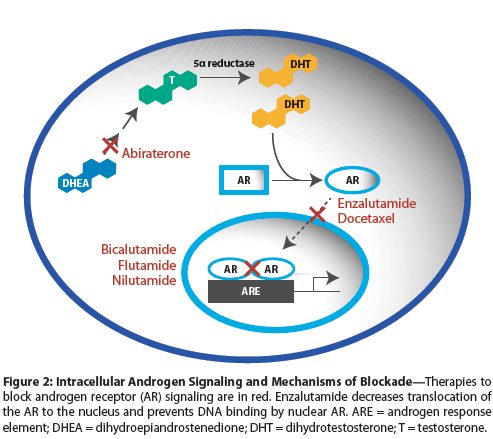
Table 1: Agents Used in Castration-Resistant Prostate Cancer
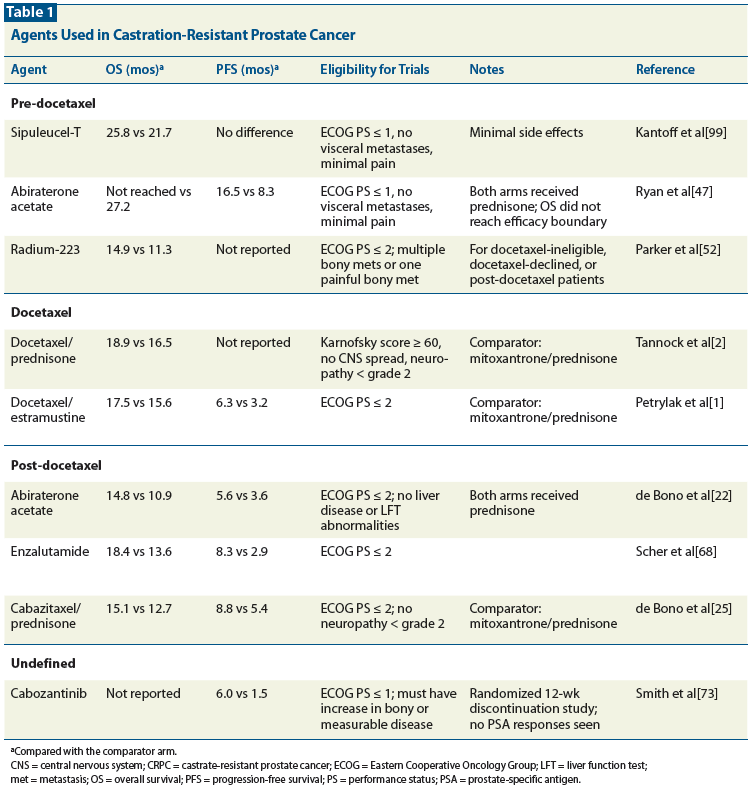
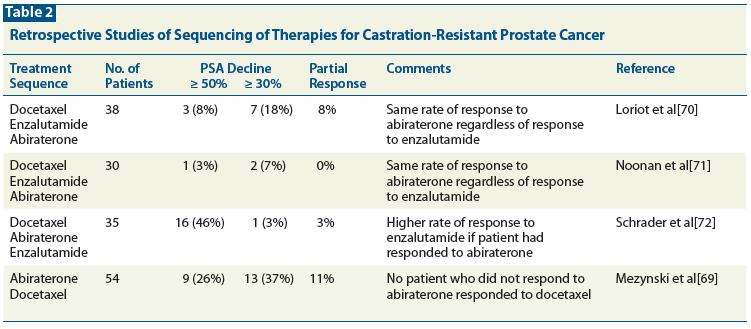
Table 2: Retrospective Studies of Sequencing of Therapies for Castration-Resistant Prostate Cancer
Ten years ago, the clinician treating metastatic castration-resistant prostate cancer (CRPC) had palliative options for treatment of symptomatic patients, such as the combination of mitoxantrone combined with prednisone, as well as isotope therapy. In 2004, docetaxel-based chemotherapy regimens were shown to provide an overall survival benefit for patients with CRPC.[1,2] Today, the prostate cancer oncologist is in the enviable position of having six US Food and Drug Administration–approved agents to choose from: immunotherapy (sipuleucel-T), hormonal therapies (abiraterone, enzalutamide), radiopharmaceuticals (radium-223), and chemotherapy (docetaxel, cabazitaxel), in addition to agents being administered in clinical trials. In general, the sequencing of these drugs is based upon the entry criteria from the phase III trials that led to their approval. Selection of treatment is based on symptoms, sites of disease (bone vs visceral) and types of prior treatment (docetaxel-ineligible vs pre-docetaxel vs post-docetaxel setting). Unfortunately, there is a lack of useful correlative biomarkers in prostate cancer to help oncologists select treatment. This problem is best illustrated in the post-docetaxel castration-resistant setting, for which there are indications to use all five other approved agents. In this review we will outline an approach to sequencing these new therapies, with particular attention paid to the biology of CRPC.
The Definition of CRPC
Castration-resistant prostate cancer (CRPC), also referred to as hormone-refractory prostate cancer, has been defined generally as prostate cancer that continues to grow after either bilateral orchiectomy or treatment with a luteinizing hormone–releasing hormone (LHRH) agonist and concurrent treatment with a nonsteroidal anti-androgen (NSAA). This type of disease used to be referred to as androgen-independent prostate cancer, but that term is used less commonly now, given our understanding that tumors remain at least partially androgen-sensitive until much later in the disease process. Furthermore, as will be discussed, androgens are present in cancer cells both in patients undergoing treatment with LHRH agonists and in those receiving treatment with NSAAs. Thus, most CRPC as currently defined depends on activity of androgens or the androgen receptor (AR). Since the term CRPC was coined, we have developed much more powerful agents to decrease testosterone levels. With the likely use of these drugs earlier in the course of disease, the term CRPC will either become outdated or eventually will refer to an entirely different set of clinical circumstances.
Androgen Signaling Basics
Our understanding of androgen signaling in prostate cancer has changed dramatically over the last decade, leading to the development of much more effective treatments. The hypothalamus produces LHRH (also called gonadotropin-releasing hormone) in pulses throughout the day. LHRH promotes production of luteinizing hormone (LH) in the pituitary. LH, in turn, acts in the Leydig cells of the testes to produce testosterone.[3] This pathway (Figure 1) produces the majority of testosterone in the body (50% to 90%, depending on age).[4] However, testosterone precursor compounds (eg, dihydroepiandrostenedione [DHEA], DHEA-sulfate [DHEA-S], and androstenedione) are produced by the adrenal glands. These precursors are taken up by the prostate, other end organs, and prostate cancer cells, where they are converted into testosterone and its more potent metabolite, dihydrotestosterone (DHT).[5-8]
The AR is a transcription factor found in the cytoplasm when it is not bound by ligand. DHT binding to the AR causes release of heat shock proteins, AR conformational change, and translocation of the AR to the nucleus. In the nucleus, the AR initiates transcription of genes that promote survival and proliferation, and of markers such as kallikrein-related peptidase 3 (KLK3), which encodes prostate-specific antigen (PSA).[9,10] Transcriptional activation by the AR is the central driver of early prostate cancers and probably of most advanced prostate cancers.
Decreasing circulating testosterone has been the major strategy for treatment of metastatic prostate cancer for over half a century. Bilateral orchiectomy was used until LHRH agonists became available (leuprolide, goserelin, triptorelin, buserelin). An LHRH antagonist (degarelix) is also approved in this setting.[11,12] Both surgical and medical castration dramatically reduce the amount of testosterone and almost always cause shrinkage of tumors, as well as decreases in the amount of circulating PSA. As noted, because of peripheral production of testosterone precursors that are metabolized to testosterone in target tissues, orchiectomy and LHRH agonist/antagonist treatment do not eliminate testosterone completely.
Given the availability of testosterone to cancer cells in patients on LHRH agonist monotherapy, LHRH agonists are combined with an NSAA AR inhibitor such as bicalutamide, flutamide, or nilutamide at first to prevent a flare reaction. A meta-analysis concluded that continuation of antiandrogens, termed combined androgen blockade (CAB), results in a 5% overall survival (OS) advantage over LHRH monotherapy, albeit with some decrement in quality of life.[13]
Initial Treatments: Inhibition of Androgen Signaling
When PSA rises or imaging studies demonstrate disease progression in patients on an LHRH agonist /antagonist or CAB, the first step in disease management is to assess whether the patient has castrate levels of circulating testosterone. Normal testosterone levels are > 300 ng/dL. Castrate levels are defined as being ≤ 50 ng/dL, although this definition is based on testing using outdated technology.[14] Furthermore, data indicate that even levels of 20 ng/dL are high enough to drive prostate cancer growth.[15-18] Testing by mass spectrometry is the gold standard, although radioimmunoassay (RIA) and chemiluminescent (CL) assays are used most commonly. Because RIA and CL assays have a high degree of variability, their results should be interpreted with caution.[14]
Most patients will achieve very low levels of testosterone (< 20 ng/dL). For patients who do not reach castrate levels of testosterone initially, or for those with castrate levels initially who then have rising testosterone, our practice is to switch from an LHRH agonist to the LHRH antagonist degarelix. Responses to degarelix have been reported in patients with noncastrate testosterone levels during treatment with an LHRH agonist.[19]
Prior to the approval of abiraterone acetate in pre-docetaxel patients, men with disease progression on LHRH monotherapy were treated most commonly by continuing the LHRH agonist and adding an NSAA. The addition of an NSAA has been shown to produce objective responses, reduce PSA level, and decrease pain,[20,21] but no OS increase has ever been demonstrated. Unfortunately, responses to NSAAs are limited; response rates are approximately 30% and usually last 3 to 5 months, although occasional durable responses lasting several years are seen.
When disease progression occurs in patients on CAB, the NSAA can be withdrawn or changed to a different NSAA. Similar to adding NSAAs, withdrawal results in responses in 20% to 40% of patients, and these usually last for several months.[22-29] When NSAAs bind to the AR, they cause it to translocate to the nucleus and bind to DNA as androgens do, but initially they repress transcription.[30-32] Studies in prostate cancer cell lines show that when the levels of AR are elevated, NSAAs become agonists and promote transcription of AR-responsive genes.[31,32]
Thus, NSAAs can become AR agonists; withdrawal probably results in exit of most of the ARs from the nucleus and decreased AR signaling. Alternatively, switching to a different NSAA results in response rates and median times to progression similar to those associated with NSAA withdrawal.[33-36] It is possible that this occurs because differences in AR-binding affinities among the three available NSAAs alters AR activity.[37,38]
One other hormonal manipulation, oral ketoconazole, has been used for more than 30 years.[39] Ketoconazole inhibits CYP17, which catalyzes two reactions required for testosterone production.[40] Thus, ketoconazole can lower the level of testosterone still remaining in a patient receiving LHRH agonists. The response rate (approximately 30%) and time to progression (about 3 months) are similar to results seen with NSAAs. Soon after ketoconazole is used, patients’ testosterone levels rise dramatically, resulting in loss of efficacy.[41] In a randomized trial comparing treatment with ketoconazole vs antiandrogen withdrawal, no survival benefit was noted. The prechemotherapy use of ketoconazole has declined significantly with the approval of abiraterone acetate in the pre-docetaxel setting. Ketoconazole is still an appropriate choice for patients who cannot afford abiraterone acetate or who have nonmetastatic CRPC.â©
Prechemotherapy Treatments
Sipuleucel-T
Sipuleucel-T is the first cell-based immunotherapy to improve OS in any carcinoma in a phase III study. In this treatment, the patient’s own mononuclear cells (including dendritic cells, the major antigen presenting cells of the immune system) are harvested by leukapheresis, incubated with a prostatic acid phosphatase (PAP)-granulocyte macrophage colony-stimulating factor (GM-CSF) fusion protein, and then re-infused into the patient.[42] The treatment is repeated three times every 2 weeks. The rationale for the treatment is to expose dendritic cells to PAP in the presence of growth factor, causing them to activate the immune system to attack cells expressing PAP (ie, prostate and prostate cancer cells) upon reinfusion. US Food and Drug Administration (FDA) approval was granted after the phase III IMPACT study (Identification of Men with a genetic predisposition to ProstAte Cancer: Targeted screening in men at higher genetic risk and controls) demonstrated a 4.1-month OS advantage of treatment with sipuleucel-T over placebo.[43] Among the 512 men on the study, 84.5% had not received docetaxel prior to treatment with sipuleucel-T. In this study, 50.7% of patients had only bony disease and 41.9% had both bony and soft-tissue disease. Patients were either asymptomatic or minimally symptomatic (with the latter defined as Eastern Cooperative Oncology Group performance status [ECOG PS] ≤ 1 with none of the following: pathologic bone fractures, visceral metastases, recently initiated treatment with bisphosphonates, and spinal cord compression). Use of glucocorticoids was also excluded, based on their potential immunosuppressive effects. Despite the reported survival advantage, no progression-free survival (PFS) advantage, PSA response, or measurable disease response was seen with sipuleucel-T. Nevertheless, PSA responses were seen in earlier studies, as was a single documented complete response.[44] It is postulated that this disconnect between PFS and OS may be due in part to delayed or prolonged activation of the immune system. Given this concept, sipuleucel-T is most appropriate for patients with slowly rising PSA levels and disease that is not seen to be increasing rapidly on CT or bone scanning. Trials are being designed to broaden indications for its use to patients with castration-resistant, biochemically relapsed prostate cancer.
Abiraterone acetate
Abiraterone acetate provided a major breakthrough in prostate cancer, both clinically and conceptually. As previously noted, abiraterone inhibits both enzymatic activities of the CYP17 enzyme: the 17α-hydroxylase and 17,20-lyase functions. These reactions are required to produce DHEA, DHEA-S, and androstenedione in the adrenal glands, which are converted to testosterone in the prostate or in prostate cancer cells. Thus, abiraterone acetate dramatically reduces the amount of testosterone in cancer cells, even in patients already producing no testicular androgen (ie, orchiectomized patients or those taking LHRH agonists).
Multiple studies,[45-47] including a large randomized placebo-controlled trial,[47] have shown impressive responses to abiraterone in patients with disease progression on LHRH agonists. The latter study[47] was stopped early because a planned interim analysis demonstrated significant PFS and OS advantages in the abiraterone acetate arm (Table 1). Regarding results of treatment with abiraterone vs placebo, the median OS was not reached with abiraterone vs 27.2 months with placebo, and the PFS was 16.5 months vs 8.3 months, respectively. Notably, for OS, a prespecified significance boundary was not reached, however the abiraterone treatment effect was favorable across all prespecified subgroups. In multiple other measures tested, treatment with abiraterone and prednisone was superior to treatment with prednisone alone (in terms of outcomes as assessed by Response Evaluation Criteria In Solid Tumors [RECIST], time to increase in pain, time to initiation of cytotoxic chemotherapy, PSA response, time to PSA progression, time to decline in functional status, etc).
A previous randomized controlled trial in patients who had already received docetaxel also demonstrated an OS advantage[22] and will be discussed later in this article.
Radium 223-dichloride
Radiopharmaceuticals have been used for pain control in prostate cancer for many years. Samarium 153 ethylenediamine tetramethylene phosphonate (153Sm-EDTMP), rhenium-186 hydroxyethylidenediphosphonate (186Re-HEDP), and strontium-89 chloride (89SrCl), are compounds that contain beta-emitting radioisotopes of samarium, rhenium, and strontium, respectively. These compounds home to areas of metabolic bone turnover, causing DNA and oxidative damage to metastatic cells at those sites. All have been shown to decrease pain from bony metastases, but evidence for an OS advantage is limited to one small phase II study.[48] These drugs are limited in efficacy because of dose-limiting bone marrow toxicity. Beta particles penetrate between 0.5 mm and 2.5 mm into soft tissue.[49] Radium-223 dichloride (Ra-223) is an alpha-emitting isotope that also targets and attacks bony disease in the same way. However, the tissue penetration of Ra-223 is only between 2 and 10 cell diameters and causes very little bone marrow toxicity. A small phase II study of 64 docetaxel-pretreated patients randomized to Ra-223 or placebo not only demonstrated a benefit in bone pain, but also demonstrated an OS advantage.[50,51] In the large phase III study comparing Ra-223 to placebo in patients with bony metastases but no visceral metastases (the Alpharadin in Symptomatic Prostate Cancer Patients [ALSYMPCA] trial),[52] OS and time to first skeletal-related event were significantly longer in the Ra-223 arm. This patient population included patients who had received docetaxel (57%) as well as those who were ineligible to receive docetaxel or declined to be treated with it (43%).
Initial Chemotherapy
Docetaxel
The first therapies with proven survival advantages in prostate cancer contained the taxane docetaxel. Taxanes inhibit microtubule breakdown, resulting in apoptosis, although recent evidence suggests that in prostate cancer cells they can inhibit nuclear translocation and expression of the AR.[53,54] In 2004, two studies demonstrated OS advantages in patients treated with docetaxel-containing regimens.[1,2] In the TAX 327 study, 1,006 men with hormone-refractory prostate cancer were randomly assigned to one of three treatment arms: mitoxantrone, docetaxel given every 3 weeks, or docetaxel given weekly. In previous studies mitoxantrone was shown to have no effect on survival (although it did palliate symptoms), so it was used as a comparator arm.[55-59] OS in both docetaxel containing arms was improved compared with the mitoxantrone arm, although only the difference between 3-week docetaxel dosing and mitoxantrone reached statistical significance. Additionally, pain, quality of life, and PSA responses were significantly improved. In the Southwestern Oncology Group (SWOG) 99-16 study, 770 men with hormone-refractory prostate cancer were randomly assigned to treatment with docetaxel and estramustine or mitoxantrone and prednisone every 3 weeks. Similar to the TAX 327 results, OS, time to progression, and PSA responses were significantly better in the docetaxel-containing arm. Owing to the similarity between the results of the two studies and the comparative ease of giving prednisone (as opposed to estramustine), docetaxel/prednisone with every-3-week dosing of docetaxel has become the standard of care.
Patient Care Following Initial Chemotherapy
After the introduction of docetaxel-based chemotherapy, there was a several-year delay in the appearance of new therapies for CRPC. However, work was progressing on several fronts concurrently, with all of these efforts aimed at those patients who had progressed on docetaxel. These included studies of abiraterone acetate, radium 223-dichloride (both discussed previously in this article), enzalutamide, and cabazitaxel. None of these agents have been directly compared in their respective treatment settings. Thus, there are no evidence-based guidelines available for how to sequence these treatments.
Abiraterone
As noted, abiraterone acetate was originally shown to benefit patients whose disease had progressed on docetaxel.[22,60,61] In a large trial, 1,195 men were randomized in a 2:1 fashion to receive prednisone with abiraterone or with a placebo.[22] At a planned interim analysis, patients who received abiraterone had an OS advantage (14.8 months vs 10.9 months) and the study was stopped. Patients in the abiraterone-containing arm also demonstrated PFS and PSA response improvements over the placebo arm. Thus, high-level evidence exists for the use of abiraterone acetate before or after docetaxel chemotherapy.
Cabazitaxel
Cabazitaxel is a potent tubulin-binding taxane with activity against docetaxel-resistant cancer cell lines.[25] In a phase III study, 755 men with prostate cancer that had progressed after treatment with docetaxel were randomized to cabazitaxel or mitoxantrone. OS and PFS were significantly better in patients treated with cabazitaxel, as was PSA response rate (39.2% vs 17.8% with mitoxantrone).
Enzalutamide
Enzalutamide is a second-generation AR antagonist with significantly more potent binding to the AR than the first-generation antagonists (bicalutamide, flutamide, nilutamide).[62] In addition, its function is qualitatively different from that of the older drugs. Enzalutamide-bound AR mostly remains cytoplasmic, whereas the older drugs actually cause nuclear translocation (as discussed previously; see Figure 2). In addition, enzalutamide-bound AR that does translocate to the nucleus does not appear to bind to DNA and so is inactive.[62]
After encouraging results in a phase I/II trial,[63] enzalutamide was analyzed in a randomized placebo-controlled phase III trial in men who had received docetaxel. In that trial, median OS was 18.4 months vs 13.6 months for enzalutamide vs placebo, respectively. Other secondary endpoints were all improved with enzalutamide: PSA response, time to PSA progression, PFS, quality of life, time to skeletal-related events, and soft-tissue response rate. Notably, a small percentage of patients (0.6%) on the enzalutamide arm had seizures.
Radium-223
As noted above, the phase III ALSYMPCA trial, which showed an OS advantage for Ra-223 over placebo, enrolled patients with metastatic prostate cancer who had or had not received docetaxel. The hazard ratio for death in those two groups was almost identical (0.71 vs 0.74%, respectively).[52]
Sequencing of Therapies
Treatment prior to docetaxel
Given the lack of randomized trial data to guide rational or biologically based sequencing of therapies, treatment in asymptomatic or minimally symptomatic patients is selected based upon rapidity of disease progression and toxicity of treatment. Given the fact that there are three clinical trials of newer agents (sipuleucel-T, abiraterone/prednisone, Ra-223) demonstrating improvement in PFS or OS in patients with metastatic disease that is progressing on androgen ablation, the addition of first-generation NSAAs has generally fallen out of favor. In a patient with slowly progressive disease, sipuleucel-T is a reasonable first treatment option, based upon its lack of toxicity and the fact that the current FDA label requires that patients be off systemic corticosteroids for 1 month prior to treatment.
Considerable controversy has arisen over the role of corticosteroids and potential interference with sipuleucel-T activity. A recently reported randomized phase II study[64] demonstrated no difference in immune function in patients who were treated with abiraterone/prednisone either concomitantly with or post completion of treatment with sipuleucel-T. Although survival differences were not a primary endpoint of this study, lack of difference in immune parameters provides some support for the early or concomitant use of abiraterone in this patient population. A similar study is now being designed that will evaluate immune parameters associated with early vs delayed enzalutamide.
In a patient with rapid asymptomatic disease progression, abiraterone/prednisone is a reasonable first treatment option, particularly in patients who have had a long duration of response to first-line hormonal therapy. With FDA approval of abiraterone acetate in chemotherapy-naive patients, the use of ketoconazole appears to be limited to patients who have nonmetastatic castrate-resistant disease or those with metastatic disease who cannot afford treatment with abiraterone.
Ra-223 is a good choice for patients with significant pain from multiple bony lesions, particularly if these metastases are too numerous for local radiation therapy to be feasible. Data from the ALSYMPCA trial suggest that hematologic toxicity is not significantly worse in patients who subsequently receive docetaxel.[65]
Last, combining LHRH agonists with either potent CYP17A inhibitors like abiraterone acetate or AR blockers like enzalutamide are either in clinical trials or in the planning stages for trials. Given the slight survival advantage of CAB over LHRH agonist monotherapy, it seems likely that these combinations would be even more efficacious.
Post-docetaxel/pre-abiraterone therapy
In patients with disease that is progressing on docetaxel and prednisone who have not yet been treated with abiraterone, there are four therapies that provide survival advantages of similar magnitude: abiraterone, enzalutamide, cabazitaxel, and Ra-223. The eligibility requirements for these trials were quite similar, so their patient populations probably were also quite similar. Thus, the major factors in choosing the order of these drugs are the side-effect profiles of the specific agents.
After docetaxel fails, either abiraterone or enzalutamide is probably the best choice for therapy, based on patterns of toxicity. Both docetaxel and cabazitaxel treatment can result in peripheral neuropathy, as well as fatigue, and sequential use can exacerbate pre-existing neuropathy. Although no comparative data exist, one could expect less fatigue and cytopenias with abiraterone or enzalutamide than with a second taxane in a patient who has already been treated with a significant amount of docetaxel. How to choose between abiraterone and enzalutamide is unclear, although many patients are relatively intolerant of glucocorticoids (eg, patients with diabetes, patients with psychiatric issues) and enzalutamide does not require their use. In fact, a retrospective analysis of the AFFIRM trial demonstrates that corticosteroid use is an independent poor prognostic factor in patients treated with enzalutamide.[66] Although there have been anecdotal reports of patients being treated with abiraterone without steroids, current labeling for abiraterone requires glucocorticoid administration.[67] Alternatively, enzalutamide should not be used in a patient with a seizure history, since seizures were observed in the trials of enzalutamide.[63,68] Once abiraterone or enzalutamide are no longer effective, cabazitaxel is a logical choice, followed by the other hormonal therapy (ie, enzalutamide if abiraterone was used first and vice versa if enzalutamide was used first). Alternatively, if disease progression is primarily in the bones, Ra-223 is an excellent option, given the lack of almost any side effects from treatment.
Post-docetaxel/post-abiraterone therapy
Most patients in the future will have received abiraterone acetate prior to treatment with docetaxel. In these patients, after they progress on docetaxel, the choice is among cabazitaxel, enzalutamide, and Ra-223. The same rationale described above for ordering therapies after docetaxel can be used here, the only difference being the omission of abiraterone. However, the trials that showed the effectiveness of these agents were not done in patients pre-treated with abiraterone. Thus, it is unknown how effective they are in this patient population. In fact, several retrospective studies suggest that each of these therapies is far less effective after treatment with one of the others. One study that analyzed response to docetaxel in patients previously treated with abiraterone found the PSA response rate to be 26%,[69] which is lower than the 45% to 50% response rate originally seen in the phase III studies of docetaxel.[1,2,69] Median OS was only 12.5 months, compared with OS outcomes of 17.5 to 18.9 months reported in the phase III trials. Three studies of patients who received docetaxel followed by either enzalutamide and then abiraterone, or these agents given in the reverse order, only showed minimal responses to the last therapy administered (Table 2).[70-72] This phenomenon should not be surprising since abiraterone inhibits AR signaling by decreasing the amount of testosterone in the cancer cells, whereas enzalutamide also inhibits AR signaling but does so through direct inhibition of the receptor protein. Depending on the mechanism of resistance that develops, the cancer might be resistant to both drugs (as will be described later in this article). Nonetheless, since some significant responses occur, it is reasonable to try each of these agents.
Future Directions
New agents: cabozantinib
Although still in clinical trials, cabozantinib (also called XL184), a dual inhibitor of mesenchymal-epithelial transition (MET) factor and vascular endothelial growth factor receptor 2 (VEGFR2), has shown activity in prostate cancer in a phase II discontinuation trial.[73] In this study 46% of patients had received chemotherapy previously, and 87% of patients had bony disease. The trial was stopped early because of evidence of activity with cabozantinib. All patients received 12 weeks of cabozantinib; 14 patients then continued to be treated with the drug, and 17 were randomized to receive a placebo. The median PFS for men who continued to receive cabozantinib was 23.9 weeks, compared with 5.9 weeks for those who received placebo. A total of 171 patients received 12 weeks of cabozantinib. Based on the PFS difference found in the discontinuation trial, a number of post-hoc analyses were performed for hypothesis generation. Investigators found that 68% of evaluable patients had a decrease in bony disease on bone scan, 67% had a decrease in pain, and 56% had a decrease in opiate use. PSA changes, however, did not correlate with response. If the ongoing phase III studies show an OS advantage for cabozantinib, then the sequencing of therapeutic agents for patients with advanced prostate cancer will only become more complex.
A large number of other agents are in randomized trials in this setting, but efficacy data have not yet been reported; therefore, these drugs are outside of the scope of this review.
Combining therapies
The results with docetaxel-based combination therapy have been disappointing. Despite having biological rationales and promising preclinical data, attempts to combine docetaxel with bone-targeted agents (atrasentan,[74] dasatinib, and ZD4054[75]), anti-angiogenesis agents (bevacizumab,[76] aflibercept,[77] lenalidomide,[78] and vitamin D (calcitriol, DN-101)[79]) have all failed to demonstrate a survival benefit. One possible reason for failure in these trials is that the aforementioned agents had modest clinical activity as single agents.[80-82]
Now that we have multiple active agents with different mechanisms of action, the challenge is to generate rational combinations without overlapping toxicities. For example, although abiraterone and enzalutamide dramatically decrease AR signaling, possibly such signaling could be decreased even more by combining these drugs. Such a study is now underway (National Cancer Institute clinicaltrials.gov ID: NCT01650194).
Alternatively, combining agents that target different pathways could increase effectiveness without necessarily increasing toxicity, in a manner analogous to that seen in combination chemotherapy. To that end, trials are either being designed or are ongoing using such approaches. Combination studies have evaluated the safety of docetaxel combined with TAK700 (a CYP17A inhibitor currently in clinical trials),[83] enzalutamide,[84] or Ra-223.[85] A study is currently analyzing the safety of combined treatment with cabazitaxel and abiraterone.[86] Sequential vs combined sipuleucel-T and abiraterone is currently under investigation,[64] and a randomized trial of early vs delayed enzalutamide, focused on evaluating immune markers, is planned in patients treated with sipuleucel-T. A randomized phase II study combining Ra-223 with enzalutamide or abiraterone/prednisone is planned.
Use of biomarkers
In an ideal world, we could perform large randomized studies to decide which sequence of therapies to use for men with CRPC. Of course, such studies would be lengthy, prohibitively expensive, and almost certainly not the best use of resources. With the use of biomarkers, however, it might be possible to distinguish among therapies that will benefit specific patients, although to date no biomarkers have been able to do this. For example, there are a number of ways in which prostate cancer cells can become resistant to androgen blockade. Some of these mechanisms remain responsive to certain hormonal therapies while others do not. Examples include:
Increased AR expression. This phenomenon causes resistance to NSAAs but not necessarily to abiraterone or enzalutamide.[87]
Increased expression of CYP17A. In tumor xenografts, increased CYP17A expression causes resistance to abiraterone.[88] This mechanism of resistance would have far less of an effect on enzalutamide.
AR mutation. Several classes of AR mutants have been described. Mutations that alter the binding of NSAAs were found in a small percentage of prostate cancers.[89] These mutations cause resistance to NSAAs, but it is unknown how they affect other therapies. A number of different AR splice mutants have been described in cell lines and in human tumors that activate AR transcription independent of androgen binding.[90-93] Theoretically, all these mutant receptors will be resistant to abiraterone. A subset of these mutant ARs have abnormal or absent ligand-binding domains, and many of those will also be resistant to treatment with enzalutamide.[94]
Activation of additional pathways to activate the AR. A number of accessory pathways (eg, interleukin (IL)-6, human epidermal growth factor receptor 2 [HER-2]/neu) have been shown to activate the AR in cell lines in the presence of very small amounts of, or the complete absence of, androgen.[95,96] Cancers harboring these activated pathways presumably would be resistant to all hormonal therapies.
Activation of AR-independent pathways. A few such molecular pathways have been identified (eg, Akt signaling[97]), and undoubtedly others will be discovered in the coming years. Cancers in which these pathways were active also would be resistant to hormonal therapies. At some point, analysis of tumors by sequencing, immunohistochemistry, or fluorescence in situ hybridization (FISH) might be used to determine whether any of these mechanisms were at work, and if so, whether they might change management. Implicit in this approach is that tissue from growing tumors will be
assessed throughout the course of the patient’s disease; however, studies have shown that circulating tumor cells can be analyzed by these methods, which might obviate the need for repeated biopsies.[98]
Conclusion
It is an incredibly exciting time to be a prostate cancer oncologist. For the first time ever, we have multiple effective agents available to our patients with metastatic disease. At the same time, however, there are enormous gaps in our understanding of how to use these agents. It is likely that the way we practice prostate cancer oncology will be dramatically different in a few years. Just as molecular studies have been the basis of many of these new therapies, it is hoped that molecular research will clarify clinical practice and improve the lives of men with advanced prostate cancer.
Disclosures:
Dr. Petrylak receives consultant fees from Bayer, Bellicum, Dendreon, Sanofi-Aventis, Johnson & Johnson, Exelixis, Ferring, Medivation, Millennium, and Pfizer; and receives grant support from Celgene, Dendreon, Johnson & Johnson, Millennium, Oncogenix, and Progenics. Dr. Hurwitz has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513-20.
2. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-12.
3. Griffin JE, Ojeda SR (editors). Textbook of endocrine physiology. New York: Oxford University Press; 2004.
4. Labrie F, Bélanger A, Luu-The V, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322-8.
5. Labrie F, Bélanger A, Simard J, et al. DHEA and peripheral androgen and estrogen formation: intracrinology. Ann N Y Acad Sci. 1995;774:16-28.
6. Geller J, Albert J, Loza D. Steroid levels in cancer of the prostate-markers of tumour differentiation and adequacy of anti-androgen therapy. J Steroid Biochem. 1979;11:631-6.
7. Titus MA, Schell MJ, Lih FB, et al. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653-7.
8. Dillard PR, Lin M-F, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115-20.
9. Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276-308.
10. Murtha P, Tindall DJ, Young CY. Androgen induction of a human prostate-specific kallikrein, hKLK2: characterization of an androgen response element in the 5' promoter region of the gene. Biochemistry. 1993;32:6459-64.
11. Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531-8.
12. Crawford ED, Tombal B, Miller K, et al. A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J Urol. 2011;186:889-97.
13. Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2007 update of an American Society of Clinical Oncology Practice Guideline. J Clin Oncol. 2007;25:1596-1605.
14. Rove KO, Debruyne FM, Djavan B, et al. Role of testosterone in managing advanced prostate cancer. Urology. 2012;80:754-62.
15. Oefelein MG, Feng A, Scolieri MJ, et al. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56:1021-4.
16. Morote J, Orsola A, Planas J, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178:1290-5.
17. Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int. 2010;105:648-51.
18. Pickles T, Hamm J, Morris WJ, et al. Incomplete testosterone suppression with luteinizing hormone-releasing hormone agonists: does it happen and does it matter? BJU Int. 2012;110:E500-7.
19. Raddin RS, Walko CM, Whang YE. Response to degarelix after resistance to luteinizing hormone-releasing hormone agonist therapy for metastatic prostate cancer. Anticancer Drugs. 2011;22:299-302.
20. Labrie F, Dupont A, Giguere M, et al. Benefits of combination therapy with flutamide in patients relapsing after castration. Br J Urol. 1988;61:341-6.
21. Kucuk O, Fisher E, Moinpour CM, et al. Phase II trial of bicalutamide in patients with advanced prostate cancer in whom conventional hormonal therapy failed: a Southwest Oncology Group study (SWOG 9235). Urology. 2001;58:53-8.
22. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
23. Kelly WK, Scher HI. Prostate-specific antigen decline after antiandrogen withdrawal: the flutamide withdrawal syndrome. J Urol. 1993;149:607-9.
24. Scher HI, Kelly WK. Flutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancer. J Clin Oncol. 1993;11:1566-72.
25. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147-54.
26. Small EJ, Srinivas S. The antiandrogen withdrawal syndrome. Experience in a large cohort of unselected patients with advanced prostate cancer. Cancer. 1995;76:1428-34.
27. Figg WD, Sartor O, Cooper MR, et al. Prostate-specific antigen decline following the discontinuation of flutamide in patients with stage D2 prostate cancer. Am J Med. 1995;98:412-14.
28. Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22:1025-33.
29. Sartor AO, Tangen CM, Hussain MHA, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group trial (SWOG 9426). Cancer. 2008;112:2393-2400.
30. Masiello D, Cheng S, Bubley GJ, et al. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J Biol Chem. 2002;277:26321-6.
31. Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33-9.
32. Baek SH, Ohgi KA, Nelson CA, et al. Ligand-specific allosteric regulation of coactivator functions of androgen receptor in prostate cancer cells. Proc Natl Acad Sci USA. 2006;103:3100-5.
33. Joyce R, Fenton MA, Rode P, et al. High dose bicalutamide for androgen independent prostate cancer: effect of prior hormonal therapy. J Urol. 1998;159:149-53.
34. Kassouf W, Tanguay S, Aprikian AG. Nilutamide as second line hormone therapy for prostate cancer after androgen ablation fails. J Urol. 2003;169:1742-4.
35. Davis NB, Ryan CW, Stadler WM, Vogelzang NJ. A phase II study of nilutamide in men with prostate cancer after the failure of flutamide or bicalutamide therapy. BJU Int. 2005;96:787-90.
36. Suzuki H, Okihara K, Miyake H, et al. Alternative nonsteroidal antiandrogen therapy for advanced prostate cancer that relapsed after initial maximum androgen blockade. J Urol. 2008;180:921-7.
37. Kolvenbag GJ, Furr BJ, Blackledge GR. Receptor affinity and potency of non-steroidal antiandrogens: translation of preclinical findings into clinical activity. Prostate Cancer Prostatic Dis. 1998;1:307-14.
38. Bohl CE, Gao W, Miller DD, et al. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc Natl Acad Sci USA. 2005;102:6201-6.
39. Pont A, Williams PL, Azhar S, et al. Ketoconazole blocks testosterone synthesis. Arch Intern Med. 1982;142:2137-40.
40. Yamaoka M, Hara T, Kusaka M. Overcoming persistent dependency on androgen signaling after progression to castration-resistant prostate cancer. Clin Cancer Res. 2010;16:4319-24.
41. Pont A. Long-term experience with high dose ketoconazole therapy in patients with stage D2 prostatic carcinoma. J Urol. 1987;137:902-4.
42. Kantoff P, Higano CS. Integration of immunotherapy into the management of advanced prostate cancer. Urol Oncol. 2012;30:S41-7.
43. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-22.
44. Burch PA, Croghan GA, Gastineau DA, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a phase 2 trial. Prostate. 2004;60:197-204.
45. Attard G, Reid AHM, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563-71.
46. Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481-8.
47. Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138-48.
48. Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357:336-41.
49. Pandit-Taskar N, Batraki M, Divgi CR. Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J Nucl Med. 2004;45:1358-65.
50. Nilsson S, Franzén L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587-94.
51. Nilsson S, Franzén L, Parker C, et al. Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer. 2013;11:20-6.
52. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213-23.
53. Zhu M-L, Horbinski CM, Garzotto M, et al. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992-8002.
54. Kuroda K, Liu H, Kim S, et al. Docetaxel down-regulates the expression of androgen receptor and prostate-specific antigen but not prostate-specific membrane antigen in prostate cancer cell lines: implications for PSA surrogacy. Prostate. 2009;69:1579-85.
55. Berry W, Dakhil S, Modiano M, et al. Phase III study of mitoxantrone plus low dose prednisone versus low dose prednisone alone in patients with asymptomatic hormone refractory prostate cancer. J Urol. 168:2439-43.
56. Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the Cancer and Leukemia Group B 9182 study. J Clin Oncol. 1999;17:2506-13.
57. Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756-64.
58. Osoba D, Tannock IF, Ernst DS, Neville AJ. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol. 1999;17:1654-63.
59. Ernst DS, Tannock IF, Winquist EW, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21:3335-42.
60. Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;
28:1496-1501.
61. Reid AHM, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;
28:1489-95.
62. Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787-90.
63. Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437-46.
64. Small EJ, Lance R, Redfern CH, et al. A randomized phase II trial of sipuleucel-T with concurrent or sequential abiraterone acetate (AA) plus prednisone (P) in metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol. 2013;31(suppl):abstr 5047.
65. Vogelzang NJ, Helle SI, Johannessen DC, et al. Efficacy and safety of radium-223 dichloride (Ra-223) in castration-resistant prostate cancer (CRPC) patients with bone metastases who did or did not receive prior docetaxel (D) in the phase III ALSYMPCA trial. J Clin Oncol 2013;31(suppl):abstr 5068.
66. Scher HI, Fizazi K, Saad F, et al. Association of baseline corticosteroid with outcomes in a multivariate analysis of the phase 3 AFFIRM study of enzalutamide (ENZA), an androgen receptor signaling inhibitor (ARSI). Presented at the 37th ESMO Congress; Vienna. 2012. Abstract 2887.
67. Attard G, Reid AHM, de Bono JS. Abiraterone acetate is well tolerated without concomitant use of corticosteroids. J Clin Oncol. 2010;28:e560-1.
68. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012; 367:1187-97.
69. Mezynski J, Pezaro C, Bianchini D, et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol. 2012;23:2943-7.
70. Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol. 2013;24:1807-12.
71. Noonan KL, North S, Bitting RL, et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802-7.
72. Schrader AJ, Boegemann M, Ohlmann C-H, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2013 Jul 2. [Epub ahead of print]
73. Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31:412-9.
74. Quinn DI, Tangen CM, Hussain M, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol. 2013;14:893-900.
75. Fizazi KS, Higano CS, Nelson JB, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2013;31:1740-7.
76. Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30:1534-40.
77. Tannock IF, Fizazi K, Ivanov S, et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol. 2013;14:760-8.
78. Petrylak DP, Fizazi K, Sternberg C, et al. A phase 3 study to evaluate the efficacy and safety of docetaxel and prednisone (DP) with or without lenalidomide (LEN) in patients with castrate-resistant prostate cancer (CRPC): the MAINSAIL trial. Presented at the 37th ESMO Congress; Vienna. 2012. Abstract 2462.
79. Scher HI, Jia X, Chi K, et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol. 2011;29:2191-8.
80. Nelson JB, Love W, Chin JL, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478-87.
81. Nabhan C, Petrylak DP. The role of IMiDs alone or in combination in prostate cancer. Clin Genitourin Cancer. 2012;10:141-6.
82. Miller K, Moul JW, Gleave M, et al. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:187-92.
83. Petrylak DP, Gandhi JG, Clark W, et al. Phase I results from a phase I/II study of orteronel, an oral, investigational, nonsteroidal 17,20-lyase inhibitor, with docetaxel and prednisone (DP) in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2012;30(suppl):abstr 4656.
84. Fleming M, Rathkopf D, Gibbons J, et al. J Clin Oncol. 2013. Enzalutamide in combination with docetaxel in men with prostate cancer (PC): preliminary results from a phase I study. J Clin Oncol. 2013;31(suppl 6):abstr 63.
85. Morris M, Hammers HJ, Sweeney C, et al. A phase I/IIa study of the safety and efficacy of radium-223 chloride (Ra-223) with docetaxel (D) for castration-resistant prostate cancer (CRPC) patients with bone metastases. J Clin Oncol. 2013; 31(suppl):abstr 5021.
86. Massard C, Pezaro C, Bobilev D, et al. A phase I/II study of cabazitaxel (Cbz) combined with abiraterone acetate (AA) and prednisone (P) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) whose disease has progressed after docetaxel (D) chemotherapy: preliminary results. J Clin Oncol. 2013;31(suppl):abstr 5049.
87. Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401-6.
88. Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503-13.
89. Taplin M-E, Rajeshkumar B, Halabi S, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673-8.
90. Steinkamp MP, O'Mahony OA, Brogley M, et al. Treatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapy. Cancer Res. 2009;69:4434-42.
91. Dehm SM, Schmidt LJ, Heemers HV, et al. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469-77.
92. Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci USA. 2010;107:16759-65.
93. Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;71:1656-67.
94. Hu R, Lu C, Mostaghel EA, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457-62.
95. Ueda T, Mawji NR, Bruchovsky N, Sadar MD. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem. 2002;277:38087-94.
96. Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280-5.
97. Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575-86.
98. Cann GM, Gulzar ZG, Cooper S, et al. mRNA-Seq of single prostate cancer circulating tumor cells reveals recapitulation of gene expression and pathways found in prostate cancer. PLoS One. 2012;7:e49144.
99. Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099-105.