Pancreatic Metastases From Renal Cell Carcinoma Treated With Stereotactic Body Radiation Therapy
In this edition of our ongoing series, the authors present two cases involving renal cell carcinoma patients treated with SBRT for pancreatic metastases.
Figure 1: SBRT Treatment Plans
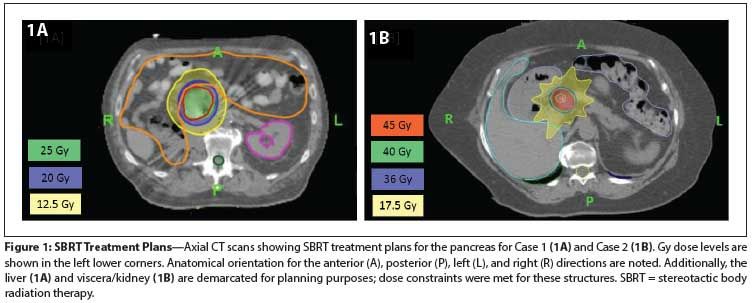
Figure 2: Case 1 CT Scan of Pancreatic Lesion Before and After SBRT
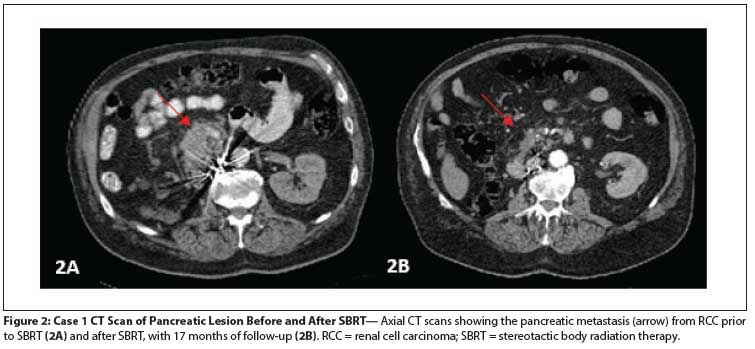
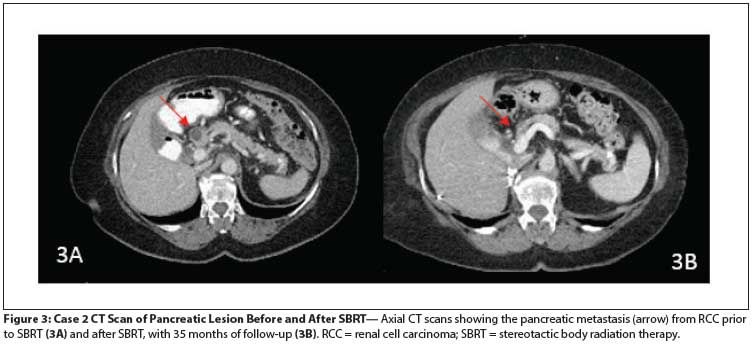
Figure 3: Case 2 CT Scan of Pancreatic Lesion Before and After SBRT
Case 1:A 75-year-old man presented with gastrointestinal bleeding 23 years after a nephrectomy for clear-cell kidney cancer (4 cm, Fuhrman grade 1/4). A CT scan showed a pancreatic head mass. An endoscopic ultrasound–guided biopsy was performed, which diagnosed metastatic renal cell carcinoma (RCC). The patient was evaluated for surgical resection, but was considered a poor candidate given his cardiovascular comorbidities and overall clinical picture. Additionally, retroperitoneal lymph nodes suspicious for metastatic disease were identified. He then started pazopanib and was initially found to have stable disease. After 11 months, he developed colonic pneumatosis and discontinued treatment because of possible drug-related bowel perforation. He then received second-line therapy with everolimus, but this agent was discontinued after 2 months due to angioedema. He was then started on sunitinib, and again needed dose reduction, this time because of increasing abdominal pain.
His pancreatic metastases had decreased from 3.8 × 7.2 cm at the start of pazopanib therapy to 2.7 × 7.1 cm at the time of pazopanib discontinuation. He had no clear evidence of active metastatic disease at that time, and because of his poor tolerance of systemic therapy, he was evaluated for stereotactic body radiation therapy (SBRT) to the pancreatic tumor. He was treated with SBRT to the head of the pancreas, 2,500 cGy in 5 fractions, 500 cGy per fraction, given daily (Figure 1A). He tolerated the SBRT well, without any adverse events. Systemic therapy was not restarted.
Seventeen months after the SBRT was completed, his pancreatic lesion had decreased in size to 1.1 × 2.3 cm, with decreased enhancement (Figure 2). The patient had minor intermittent back pain, mainly due to exercise; the pain was not believed to be related to the SBRT. Thus, with 17 months of follow-up, the treated pancreatic lesion had not progressed, and the patient was without symptoms.
Case 2: A 64-year-old woman underwent right nephrectomy of an 11.5-cm clear-cell RCC, Fuhrman grade 3, with invasion of the perinephric adipose tissue. Initial staging scans were negative for metastatic disease; however, 6 months later, metastases were found in the abdominal wall, the nephrectomy bed, and the mediastinum. The patient was started on sorafenib but experienced a significant rash that led to treatment interruption shortly after initiation. She tolerated sorafenib reintroduction, but follow-up assessments showed rapidly progressive disease. She was switched to bevacizumab but experienced bleeding from her abdominal wall tumors; therapy was discontinued soon after starting.
In light of her poor tolerance of systemic therapy, she underwent metastasectomies: open tumorectomy excision of the abdominal wall/skin tumor recurrence, tumorectomy of the renal fossa, and cryoablation of the RCC and perihilar lymphadenopathy. Shortly after these, restaging scans demonstrated innumerable bilateral lung nodules, and she was once again started on systemic therapy, this time with the experimental combination of sirolimus and erlotinib. After 2 months of therapy, imaging revealed lung and lymph node disease progression. She was then started on sunitinib therapy but had an enlarging lymph node in the aortopulmonary window. A left video-assisted thoracoscopic surgery for resection of the left aortopulmonary window metastases was performed, and the specimens obtained were consistent with RCC metastases. She resumed therapy until disease progression.
A growing subcarinal lesion and a 2.4-cm pancreatic lesion were noted on restaging CT scans. She underwent fine-needle aspiration biopsy of the pancreatic lesion and the specimen obtained was consistent with metastatic RCC. The pancreatic disease was first noted 5.5 years after the initial diagnosis. She underwent SBRT to the subcarinal lymph node and the pancreatic lesion; both were treated with 4,000 cGy in 10 fractions, at 400 cGy per fraction, given daily (Figure 1B). She had no symptoms subsequent to the SBRT treatment, and her pancreatic enzymes remained within normal limits.
After SBRT, and in light of her progressive disease, she was started on everolimus, but she developed drug-induced pneumonitis and the drug was discontinued. Pazopanib was administered, followed by axitinib, until she developed painful bone progression in the right humerus; on follow-up scans, the pancreatic lesion appeared atrophic, with a decrease in size to 10 × 6 mm and associated reduction in enhancement (Figure 3). She subsequently had a right proximal humerus resection of a 3.5-cm focus of metastatic RCC involving cancellous bone, and a prosthesis was placed. She was started on a phase I bevacizumab and programmed death ligand 1 (PD-L1) inhibitor protocol.
She later developed extensive pelvic bone disease, stopped systemic treatment, and underwent orthopedic surgery for disease stabilization before entering hospice care. With 3 years of follow-up after SBRT for her pancreatic lesion, there was no progression or development of any symptoms from this malignant deposit.
Discussion
The optimal treatment of metastatic disease to the pancreas has not been established, due to the rarity of this entity.[1] While several cancer types are known to spread to the pancreas, RCC is the most common source.[2] Surgery has been the most commonly used treatment modality, but surgery is associated with significant morbidity, and many patients are not candidates.[3] SBRT (sometimes informally called “stereotactic ablative body radiotherapy” to reflect the tumor-ablative effect[4]) is another emerging option.
Studies of surgical resection as a treatment for isolated pancreatic metastases have been promising, with 5-year survival rates of 43% to 88% reported.[3] Many patients with advanced RCC are not candidates for pancreatic surgery for medical reasons, and metastatic pancreatic lesions are frequently technically unresectable, limiting the use of this approach.[5] In this scenario, other available treatment modalities that can control metastatic disease warrant investigation. Whether SBRT is able to control pancreatic disease and provide symptom palliation has not been determined. Historically, the utility of external beam radiotherapy with standard fractionation for RCC has been questioned,[6,7] but recent reports of high rates of local control with the use of SBRT to treat RCC have led to frequent use of SBRT in this setting.[8]
Kidney cancer metastasizes to a wide range of body sites, including the viscera. Pancreas involvement of RCC occurs at a mean interval of about 10 years after nephrectomy (standard deviation = 6.5), and the two cases reported here illustrate this variability.[9] In a review of 236 cases of pancreatic metastases, 65% were symptomatic, mainly for abdominal pain (20%), gastrointestinal bleeding due to duodenal infiltration (20%), obstructive jaundice (9%), weight loss (9%), pancreatitis (3%), and diabetes (3%).[9] The patients described here experienced bleeding and pain related to pancreatic disease. The frequently symptomatic nature of pancreatic metastases typically warrants consideration of local intervention when feasible.
Outcomes in patients with pancreatic disease who do not pursue resection are difficult to interpret, given the selection bias involved in choosing candidates able to undergo surgery. In a retrospective review of isolated pancreatic metastases from clear-cell RCC, 10 patients with unresected lesions were compared with 139 patients who had undergone radical resection. The actuarial 3- and 5-year survival rates of 21% and 0%, respectively, for unresected metastases were significantly poorer (P = .038) than those for the cohort with resected lesions (78% and 72%, respectively).[9] However, as in our patients, such a resection cannot always be performed; this is especially true of elderly patients not fit for surgery and those with unresectable tumors. The need for other treatment options beyond surgery is clear. Alternative local therapy approaches to the pancreas are limited to radiation therapy and radiofrequency ablation (RFA). Although RFA has been used previously for unresectable tumors in the liver, lung, bone, adrenal glands, breast, and other sites, the potential hazards of this technique’s application to the pancreas include thermal injury to the bile duct, duodenum, transverse colon, and portal vein. Additionally, acute necrotizing pancreatitis, pancreatic fistula, or pancreatic ascites can be induced.[10-12]
RCC has been thought to be a relatively “radioresistant” tumor. This notion arose from the observation that higher doses of conventionally fractionated radiotherapy were needed to achieve the same level of clinical response achieved with lower doses in most other cancer types.[13] Preclinical data have demonstrated that the delivery of multi-session high-dose-per-fraction extracranial radiotherapy-or SBRT-has been shown to be effective in a preclinical model.[14] A recent review of the use of SBRT in patients with both primary and metastatic RCC tumors highlighted the successful clinical translation of SBRT and supported an apparent advantage for this modality over standard conventionally fractionated radiotherapy.[15] It is believed that high-dose-per-fraction radiotherapy triggers a rapid wave of endothelial apoptosis, with tumor cell death occurring 2 to 3 days later, and with the acid sphingomyelinase pathway implicated in this event; this could be a possible explanation for the superior clinical efficacy of SBRT in this setting.[16,17]
The evidence for radiation therapy in the setting of pancreatic metastases is scarce. It is limited to a small case series in which four patients received standard 2-Gy fractions of radiation combined with immunotherapy.[18] While SBRT is increasingly described as a treatment modality for primary unresectable or oligometastatic disease in RCC, there is a paucity of reports of its use for the treatment of pancreatic RCC lesions.
The largest case series of oligometastatic extracranial RCC tumors included 50 patients with 162 sites radiated, revealing a 90% overall response rate. Interestingly, in this series only one pancreatic lesion was treated. The lesion was adjacent to the stomach and duodenum, and the patient experienced a grade 5 side effect with a fatal gastric hemorrhage 4 months after SBRT.[19] The dosimetric details of that case are not included in this early report. This toxicity brings to mind a Danish study, reported around the same time, in which toxicities were observed in the treatment of primary pancreatic cancer when large volumes of bowel received a dose above 30 Gy in 3 fractions.[20]
Fortunately, these cautionary lessons were heeded by later investigators, who successfully interdigitated SBRT with systemic chemotherapy to produce low rates of bowel toxicity in regimens that achieved outcomes that compete favorably with conventionally fractionated radiotherapy for pancreatic cancer.[21-23]
In the 2010 QUANTEC (Quantitative Analyses of Normal Tissue Effects in the Clinic) study, sponsored jointly by the American Association of Physicists in Medicine and the American Society for Radiation Oncology, it was recommended that the maximum point dose to the small bowel should not exceed 30 Gy in a 3- to 5-fraction regimen.[24] This limit was respected in the first case described here; indeed, the dose employed was more conservative than is typically used for primary pancreatic cancer and was borrowed from the large number of reported experiences of preoperative radiotherapeutic treatment for rectal cancer, in which a low incidence of small bowel toxicity had been observed.[25] When there are anatomic constraints that prevent compliance with this limit, here at the University of Colorado, we generally employ an “SBRT-lite” regimen of 40 Gy in 10 fractions, as was done at the University of Rochester,[26] which permits liberalization of the maximum bowel dose constraints by virtue of the slightly more protracted fractionation schedule, yet still achieves very good durable local control for a range of target lesions.[27] This strategy was employed successfully in the second case reported here.
How best to combine SBRT with either immunotherapeutic or molecularly targeted agents remains an active area of investigation. Provocative results demonstrating an intriguing abscopal effect have been seen with the combination of SBRT and interleukin-2.[28] In other settings where a targeted agent is achieving stable disease except for limited sites of oligoprogression, the selective use of SBRT and other locally ablative therapies can eradicate resistant clonogens and prolong the opportunity to access the active therapy.[29,30]
Conclusion
With 17 and 36 months of follow-up, respectively, in our two patients, SBRT provided ongoing local control without significant concurrent or subsequent adverse events. With accumulating reports of the efficacy and tolerability of SBRT for metastatic RCC tumors at other sites, the consideration of SBRT in patients with pancreatic metastases is supported.
Acknowledgement:The authors would like to acknowledge Canales de Ayuda A.C. for their support of Dr. Bourlon’s visiting fellowship in urologic oncology at the University of Colorado Cancer Center.
Financial Disclosure:Dr. Flaig has received research support from Novartis, and his institution has received payment for clinical trial costs from Novartis, Pfizer, SmithKline Beecham, and Bristol-Myers Squibb. The other authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Zerbi A, Ortolano E, Balzano G, et al. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Ann Surg Oncol. 2008;15:1161-8.
2. Adsay NV, Andea A, Basturk O, et al. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004;444:527-35.
3. Machado NO, Chopra P. Pancreatic metastasis from renal carcinoma managed by Whipple resection. A case report and literature review of metastatic pattern, surgical management and outcome. JOP. 2009;10:413-8.
4. Loo BW Jr, Chang JY, Dawson LA, et al. Stereotactic ablative radiotherapy: what’s in a name? Pract Radiat Oncol. 2011;1:38-9.
5. Untch BR, Allen PJ. Pancreatic metastasectomy: the Memorial Sloan-Kettering experience and a review of the literature. J Surg Oncol. 2014;109:28-30.
6. Onufrey V, Mohiuddin M. Radiation therapy in the treatment of metastatic renal cell carcinoma. Int J Radiat Oncol Biol Phys. 1985;11:2007-9.
7. Halperin EC, Harisiadis L. The role of radiation therapy in the management of metastatic renal cell carcinoma. Cancer. 1983;51:614-7.
8. Stinauer MA, Kavanagh BD, Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34.
9. Sellner F, Tykalsky N, De Santis M, et al. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol. 2006;13:75-85.
10. Elias D, Baton O, Sideris L, et al. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur J Surg Oncol. 2004;30:85-7.
11. Hadjicostas P, Malakounides N, Varianos C, et al. Radiofrequency ablation in pancreatic cancer. HPB (Oxford). 2006;8:61-4.
12. Varshney S, Sewkani A, Sharma S, et al. Radiofrequency ablation of unresectable pancreatic carcinoma: feasibility, efficacy and safety. JOP. 2006;7:74-8.
13. Vaeth JM. Proceedings: Cancer of the kidney--radiation therapy and its indications in non-Wilms’ tumors. Cancer. 1973;32:1053-5.
14. Walsh L, Stanfield JL, Cho LC, et al. Efficacy of ablative high-dose-per-fraction radiation for implanted human renal cell cancer in a nude mouse model. Eur Urol. 2006;50:795-800.
15. De Meerleer G, Khoo V, Escudier B, et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 2014;15:3170-7.
16. Gulbins E. Regulation of death receptor signaling and apoptosis by ceramide. Pharmacol Res. 2003;47:393-9.
17. Garcia-Closas M, Malats N, Silverman D, et al. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet. 2005;366:649-59.
18. Saito J, Yamanaka K, Sato M, et al. Four cases of advanced renal cell carcinoma with pancreatic metastasis successfully treated with radiation therapy. Int J Clin Oncol. 2009;14:258-61.
19. Wersäll PJ, Blomgren H, Lax I, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol. 2005;77:88-95.
20. Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823-30.
21. Gurka MK, Collins SP, Slack R, et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: a pilot trial demonstrating safety. Radiat Oncol. 2013;8:44.
22. Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:735-42.
23. Tozzi A, Comito T, Alongi F, et al. SBRT in unresectable advanced pancreatic cancer: preliminary results of a mono-institutional experience. Radiat Oncol. 2013;8:148.
24. Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S101-7.
25. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827-33.
26. Milano MT, Katz AW, Schell MC, et al. Descriptive analysis of oligometastatic lesions treated with curative-intent stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1516-22.
27. Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878-86.
28. Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Sci Transl Med. 2012;4:137ra74.
29. Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807-14.
30. Gan GN, Weickhardt AJ, Scheier B, et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys. 2014;88:892-8.