Promising Early Results With Capsule Endoscopy for Colon Cancer Screening
Capsule endoscopy may eventually rival colonoscopy for the detection of colon polyps, according to the leaders of an ongoing clinical trial comparing the two technologies.
WASHINGTON-Capsule endoscopy may eventually rival colonoscopy for the detection of colon polyps, according to the leaders of an ongoing clinical trial comparing the two technologies. An interim analysis from the trial shows encouraging sensitivity and negative predictive values for detection of significant findings, compared to colonoscopy, said Jacques Deviere, MD, of Erasme Hospital, Brussels, who presented the findings at Digestive Disease Week 2007 (abstract 639c).
In capsule endoscopy, the patient swallows a pill-sized camera which then transmits several images per second as the pill traverses the digestive tract. Now mainly used for detecting causes of gastrointestinal bleeding and for irritable bowel disease, it is under study as a screening tool for colorectal cancer.
Eight European Centers
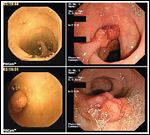
PillCam colon capsule images (left) vs colonoscopy images (right) of a pedunculated polyp in the sigmoid colon. Images courtsey of Dr. Jacques Deviere, Erasme Hospital, Brussels.
The prospective, multicenter trial, now under way at eight centers in Europe, is comparing colonoscopy to the PillCam Colon Capsule from Given Imaging, Yoqneam, Israel. The trial is designed to include 329 patients known or suspected to have a disease of the colon. Patients undergo the normal preparation for colonoscopy and then ingest the PillCam capsule along with prokinetic agents and additional small doses of laxative.
Once the capsule is excreted, the patient undergoes traditional colonoscopy. The investigators who perform the colonoscopy and review the capsule video are blinded to each other's results. With 84 patients included in the planned interim analysis, 59 (70%) were found to have at least one polyp of any size, according to colonoscopy, while 38 (45%) had a significant finding-three or more polyps or a polyp 6 mm or larger in diameter.
Capsule endoscopy detected 76% of the colonoscopy-identified polyps of any size and 79% of the cases with significant findings, Dr. Deviere reported. The specificity of PillCam was 76% (any polyps) and 78% (significant findings) of that of colonoscopy specificity.
The positive predictive value was 88% (any polyps) and 75% (significant findings) of that of colonoscopy. The negative predictive value of the test was 58% of that of colonoscopy for any polyps but 82% for significant findings.
The authors point out that compliance with traditional colorectal cancer screening recommendations is low. The ease of taking a pill could change this. "If these data are further validated at the conclusion of this study, this new noninvasive technology might challenge colonoscopy for colorectal cancer screening and polyp detection," Dr. Deviere concluded.