(S023) Stage I Lung SBRT Clinical Practice Patterns
In this retrospective study, we sought to investigate the dose prescription pattern use in the US for patients receiving SBRT.
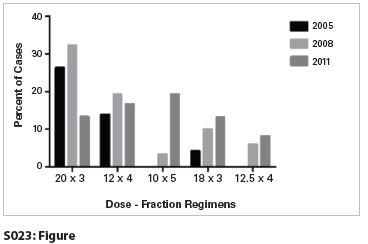
S023: Figure
Christopher D. Corso, MD, PhD, Henry S. Park, MD, MPH, Amy C. Moreno, BS, Anthony W. Kim, MD, Zain Husain, MD, James B. Yu, MD, Roy H. Decker, MD, PhD; Department of Therapeutic Radiology, Yale University
Introduction: Stereotactic body radiation therapy (SBRT) is a technique that has become increasingly utilized over the last decade in the treatment of patients with stage I non–small-cell lung cancer (NSCLC). While prior studies have suggested optimal biological effective dose (BED) versus necessary minimum BED for lung SBRT, no standardized clinical guidelines exist. In this retrospective study, we sought to investigate the dose prescription pattern use in the US for patients receiving SBRT.
Methods: Using the National Cancer Database (NCDB), adult patients aged 19 years and older with a clinical diagnosis of stage I NSCLC (cT1–2, cN0, cM0) between 2003 and 2011 whose primary treatment involved radiosurgery to the lung were identified. BED was calculated for each patient using a lung alpha/beta ratio of 10 and the recorded fraction number and total dose. Patients were excluded if their reported BED was greater than 300 or less than 10 to exclude data entry errors in the database. The most common dose-fraction prescriptions were analyzed during the years 2005, 2008, and 2011.
Results: A total of 6,324 patients were reported to have received radiosurgery to the lung, and 5,582 were analyzed as having reported BEDs within the predefined range. The overall mean and median BEDs were 130.1 and 124.8, respectively. Of these patients, 89.6% was prescribed a regimen with a BED ≥ 100. However, when confined to the three most recent years (2009–2011), the mean BED fell to 127.4, and only 74.5% of patients were prescribed a regimen with BED ≥ 100. The most common prescriptions overall were 60 Gy in 3 fractions (n = 1275, 22.8%), 48 Gy in 4 fractions (n = 937, 16.8%), 50 Gy in 5 fractions (n = 688, 12.3%), and 54 Gy in 3 fractions (n = 674, 12.1%). Analysis of the prescription trends from 2005, 2008, and 2011 revealed decreased utilization of 60 Gy in 3 fractions (26.3% in 2005 to 13.3% in 2011) and increased utilization of 50 Gy in 5 fractions (0% in 2005 to 19.3% in 2011), 54 Gy in 3 fractions (4.2% in 2005 to 13.2% in 2011), and 50 Gy in 4 fractions (0% in 2005 to 8.1% in 2011).
Conclusion: Our findings suggest that despite phase II evidence demonstrating the best published local control and overall survival to date with a regimen of 54 Gy in 3 fractions (corrected), over 25% of patients with stage I NSCLC treated with SBRT in recent years have been prescribed regimens that provide a BED of less than 100. Possible explanations for this include the increasing use of SBRT for treating centrally located tumors or tumors near organs at risk and increased adoption of SBRT by physicians who are new to the technology and, consequently, more conservative in their target prescription BED.
Proceedings of the 96th Annual Meeting of the American Radium Society - americanradiumsociety.org
Targeted Therapy First Strategy Reduces Need for Chemotherapy in Newly Diagnosed LBCL
December 7th 2025Lenalidomide, tafasitamab, rituximab, and acalabrutinib alone may allow 57% of patients with newly diagnosed LBCL to receive less than the standard number of chemotherapy cycles without compromising curative potential.