(S004) Radiation Publications Underrepresented in High-Impact General Medical and Oncology Journals
Cancer treatment studies are well represented in high-impact oncology as well as general medical journals. We sought to evaluate the distribution and characteristics of oncology studies by intervention (radiation, surgery, cytotoxic chemotherapy, or targeted/systemic agents) in six high-impact oncology and general medical journals.
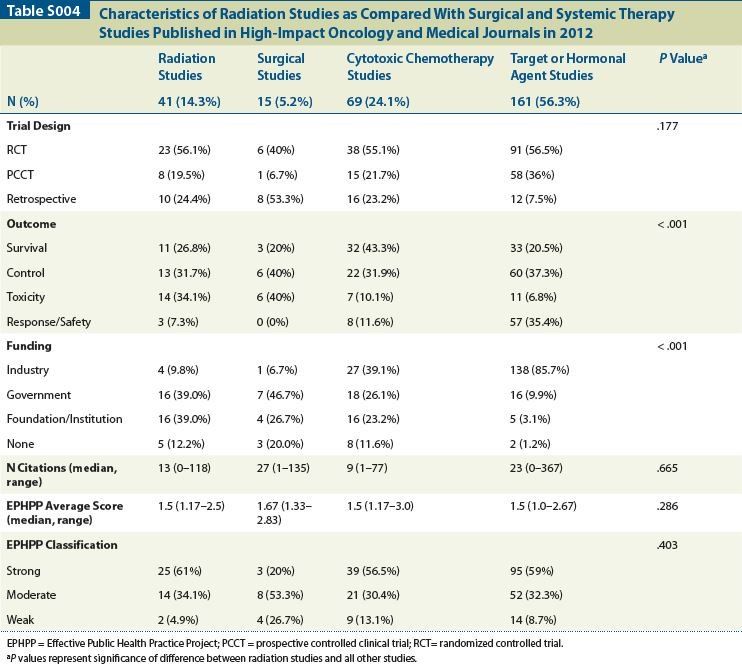
Table S004
Emma B. Holliday, MD, Awad A. Ahmed, Stella K. Yoo, Reshma Jagsi, MD, DPhil, Karen E. Hoffman, MD, MHSc, MPH; UT MD Anderson Cancer Center; Temple University Medical School; University of Michigan
Objective: Cancer treatment studies are well represented in high-impact oncology as well as general medical journals. We sought to evaluate the distribution and characteristics of oncology studies by intervention (radiation, surgery, cytotoxic chemotherapy, or targeted/systemic agents) in six high-impact oncology and general medical journals.
Methods: Research articles that were published in 2012 were identified via a commercially available online database (Scopus, Elsevier BV, NL). The journals that were included were The New England Journal of Medicine, The Lancet, The Journal of the American Medical Association, The Lancet Oncology, The Journal of Clinical Oncology, and The Journal of the National Cancer Institute. Studies were included if they evaluated a therapeutic intervention in a randomized controlled trial (RCT), prospective controlled clinical trial (PCCT), or retrospective review (RR). Intervention type, outcome measure, and number of citations per article were recorded from Scopus on September 18, 2013. Each study was scored using the Effective Public Health Practice Project (EPHPP) Quality Assessment Tool, which averages six components to provide a total numeric score from 1–3, (1 = strong, 2 = moderate, 3 = weak). Total quality rating was defined as “strong” if no individual components rated 3, “moderate” if one was rated 3, and “weak” if more than one was rated 3. Data were analyzed (SPSS version 17; Chicago, IL) using Pearson chi-square test for between-group comparisons of categorical variables and Mann-Whitney U test to compare medians for continuous variables.
Results: A total of 286 studies were included: 41 investigating radiation, 15 investigating surgery, 69 investigating cytotoxic chemotherapy, and 161 investigating targeted/other systemic therapies. There were no significant differences between radiation studies and the remaining studies in terms of distribution of trial type (P = .177); number of citations (P = .665); EPHPP average numeric score (P = .286); or designation as strong, intermediate, or weak (P = .403). When controlling for trial type in the univariate analysis, there was still no significant difference between the groups in terms of EPHPP score (P = .109). Radiation studies had lower rates of industry funding than the rest of the studies (P < .001). Additionally, more radiation studies evaluated tumor control or treatment-related toxicity, and fewer studies evaluated response/safety as endpoints (P < .001) (Table).
Conclusions: Far fewer radiation oncology studies are published when compared with studies evaluating either traditional cytotoxic chemotherapy or targeted/other systemic agents, although radiation studies that are published in the high-impact oncology and medical literature appear to have comparable quality. Further attention must be paid to identify and correct potential biases in oncology publications and to ensure that radiation oncology studies are not relegated to specialty-specific journals that fail to reach a broad audience.
Proceedings of the 96th Annual Meeting of the American Radium Society - americanradiumsociety.org