(S015) Temporal Lobe Radionecrosis After Skull Base Radiotherapy: Dose-Volume Predictors
Temporal lobe radionecrosis is a potential complication of high-dose radiation therapy for skull base tumors. The risk of radionecrosis increases with absolute doses greater than 60 Gy, but little data are available regarding potential partial volume effects when higher-dose conventionally fractionated radiotherapy is employed.
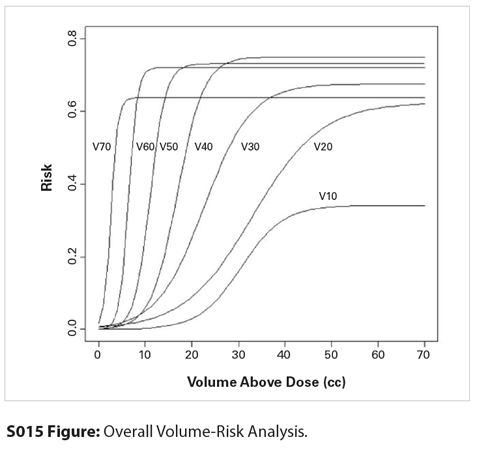
S015 Figure
Okechukwu R. Linton, MD, MBA, Mark W. McDonald, MD; Indiana University Health Proton Therapy Center, Indiana University School of Medicine
Background and Purpose: Temporal lobe radionecrosis is a potential complication of high-dose radiation therapy for skull base tumors. The risk of radionecrosis increases with absolute doses greater than 60 Gy, but little data are available regarding potential partial volume effects when higher-dose conventionally fractionated radiotherapy is employed.
Materials and Methods: Patients who were previously treated with fractionated proton therapy for skull base chordoma, chondrosarcoma, adenoid cystic carcinoma, or paranasal sinus tumors between 2005 and 2012 were analyzed, provided there was a minimum of 6 months of clinical and radiographic follow-up after treatment. The patient factors that were collected were gender, age, hypertension, diabetes, and smoking status. Treatment factors included chemotherapy utilization, whether patients’ temporal lobes had been prospectively contoured or retrospectively contoured for this review, and the absolute dose and absolute volume data for both the right and left temporal lobes, considered separately. Generalized estimating equations were used to test for the association between each patient and treatment factor and radiation necrosis, adjusting for within-subject correlation due to repeated measures. The risk of temporal lobe radionecrosis as a function of the absolute volume of a single temporal lobe irradiated was modeled using the median effective concentration (EC50) equation. The absolute volume of a temporal lobe receiving 10–70 Gy (relative biological effectiveness [RBE]) in 10-Gy (RBE) increments was analyzed (aV10, aV20, etc).
Results: Sixty-six patients and 131 temporal lobes were included in the analysis. One patient received radiation dose to only one temporal lobe due to a well-lateralized tumor. The median prescribed dose was 75.6 Gy (RBE) (range: 62–79.2 Gy [RBE]) in 1.8–2-Gy (RBE) fractions. Seven patients (11%) received concomitant chemotherapy. The median follow-up time after completion of radiation therapy was 27 months. Thirteen temporal lobes were observed to develop radionecrosis in 10 patients at a median time of 20 months after radiation. The 2-year Kaplan-Meier estimate of the risk of any-grade temporal lobe radionecrosis in this cohort was 13% (95% confidence interval [CI], 6%–20%). In the multivariable analysis, only radiation dose-volume relationships were associated with development of radionecrosis. In the EC50 model, all dose levels from 10 to 70 Gy (RBE) were highly correlated with radionecrosis, with a 15% 2-year risk of any-grade temporal lobe radionecrosis when the absolute volume of a temporal lobe receiving 30 Gy (RBE) (aV30) exceeded 16.3 cm3, aV40 >13 cm3, aV50 > 8.8 cm3, or aV60 > 5.2 cm3.
Conclusions: Dose-volume parameters are highly correlated with the risk of developing temporal lobe radionecrosis. In the setting of high-dose skull base radiotherapy, larger volumes exposed to lower doses and smaller volumes exposed to higher doses are both highly correlated with the risk of developing temporal lobe radionecrosis. Treatment planning goals should include reduction in both integral and maximal dose to the temporal lobes. The EC50 model provides suggested dose-volume temporal lobe constraints for conventionally fractionated high-dose skull base radiotherapy.
Proceedings of the 96th Annual Meeting of the American Radium Society - americanradiumsociety.org
Giredestrant Combo Yields Positive PFS in Subgroups After CDK4/6i in ER+/HER2– Breast Cancer
December 13th 2025“The magnitude of clinical benefit was clinically meaningful and consistent, and was regardless of PIK3CA mutations or alterations in the PIK3CA pathway, duration of prior CDK4/6 inhibitors, including patients who progress within 6 to 12 months, and the choice of prior CDK4/6 inhibitors,” said Hope S. Rugo, MD.