Clinical Trials in Progress: GOZILA
Nationwide Circulating-Tumor DNA–Guided Basket and Umbrella Clinical Trials for Patients With Advanced Solid Tumors (GOZILA; UMIN000029315)
Title
Nationwide Circulating-Tumor DNA–Guided Basket and Umbrella Clinical Trials for Patients With Advanced Solid Tumors (GOZILA; UMIN000029315)
Background
Marked advances in precision oncology have made genotyping mandatory for most patients with advanced cancer to ensure proper therapy selection. However, tissue-based genotyping hampers patient recruitment due to the long turnaround time, and it often fails to detect chronological tumor evolution and intratumoral genomic heterogeneity. Both of these are obstacles for accurate treatment selection.
To address these challenges, we initiated a nationwide plasma-based screening project, GOZILA, in which comprehensive circulating tumor DNA (ctDNA) sequencing was used to rapidly screen patients with advanced solid tumors for trial eligibility by identifying genomic alterations in tumor cells throughout the body. Using data from the GOZILA study, we demonstrated that ctDNA genotyping led to markedly faster turnaround time and an accelerated enrollment in targeted trials while maintaining efficacy equivalent with that of tissue-based genotyping.1
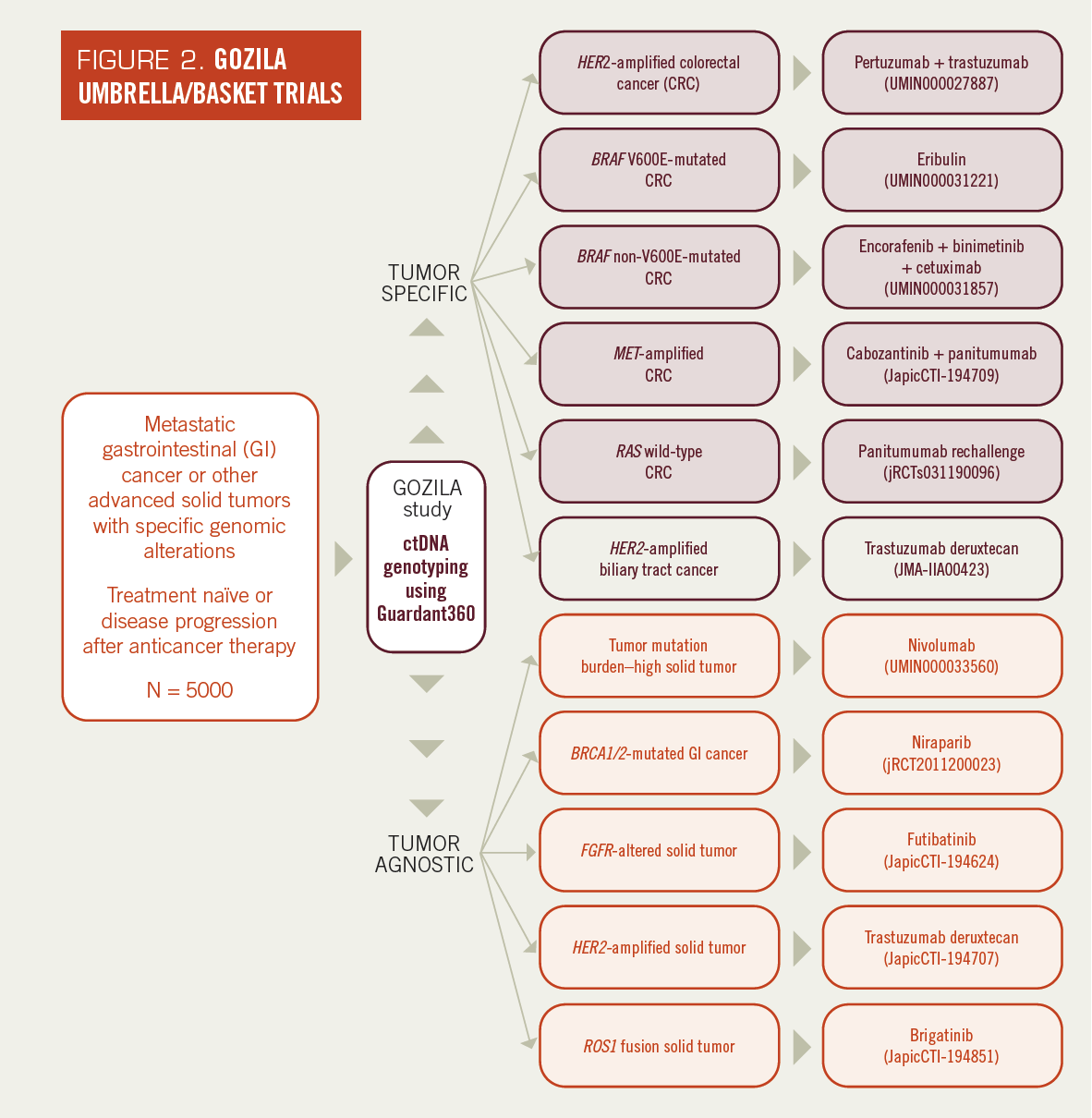
Here, we are conducting basket and umbrella-type investigator-initiated, single-arm phase 2 trials based on genomic alterations identified in the GOZILA study (Figure 2). Our goal is to determine if ctDNA genotyping can identify patients who benefit from targeted therapy. In addition, we will evaluate the efficacy of targeted therapies in GOZILA-affiliated trials compared with standard-of-care therapy in SCRUM-Japan Registry, a cohort study collecting regulatory-grade, real-world data.
Inclusion criteria
The key eligibility criteria included the following: (1) histopathologically confirmed metastatic gastrointestinal cancer or another advanced solid tumor with specific genomic alterations; (2) 20 years or older; and (3) life expectancy of at least 12 weeks. To avoid the suppression of ctDNA shedding due to chemotherapy, patients were included only if they showed disease progression during systemic chemotherapy and had not started the subsequent therapy at the time of blood sampling.
Eleven investigator-initiated trials are affiliated with the GOZILA study. Patients with a specific cancer type (tumor-specific) or any solid tumors (tumor-agnostic) and with a targeted genomic alteration identified in the GOZILA study are eligible in these trials.
Patient accrual information
Open date: Open to enrollment at National Cancer Center Hospital East in January 2018.
Accrual goal: 5000
Percent accrued: 81% accrual completed. Among affiliated clinical trials, patient accrual has been completed in TRIUMPH, BRAVERY, HERB, and TiFFANY.
Study sites
A total of 31 sites are open to accrual: National Cancer Center Hospital East, Aichi Cancer Center Hospital, National Cancer Center Hospital, National Hospital Organization Kyushu Cancer Center, Hokkaido University Hospital, Saitama Cancer Center, Kanagawa Cancer Center, Kansai Rosai Hospital, National Hospital Organization Shikoku Cancer Center, National Hospital Organization Osaka National Hospital, University of Tsukuba Hospital, Chiba Cancer Center, Kyorin University Hospital, Kindai University Hospital, Kyushu University, St Marianna University School of Medicine, Osaka University, Cancer Institute Hospital of Japanese Foundation for Cancer Research; Kobe City Medical Center General Hospital, Osaka Medical College Hospital, Gifu University, Kanazawa University, Shizuoka Cancer Center, Kagawa University Hospital, Keio University Hospital, Saitama Medical University International Medical Center, Shimane Prefectural Central Hospital, Kansai Medical University Hospital, Kyoto Katsura Hospital, Osaka International Cancer Institute, Osama General Medical Center
FIGURE 2. GOZILA Umbrella/Basket Trials
Contact Information
Yoshiaki Nakamura, MD, PhD
National Cancer Center Hospital East, 6-5-1 Kashiwanoha,
Kashiwa, Chiba 277-8577, Japan
yoshinak@east.ncc.go.jp
Phone: +81 4 7133 1111
Fax: +81 4 7134 6928
Takayuki Yoshino, MD, PhD
National Cancer Center Hospital East, 6-5-1 Kashiwanoha,
Kashiwa, Chiba 277-8577, Japan
tyoshino@east.ncc.go.jp
Phone: +81 4 7133 1111
Fax: +81 4 7134 6928
Reference
Nakamura Y, Taniguchi H, Ikeda M, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med. 2020;26(12):1859-1864. doi:10.1038/s41591-020-1063-5.

Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.