The Growing Importance of Skin Toxicity in EGFR Inhibitor Therapy
The review by LoRusso is a critical update to what could be considered the most significant dermatologic toxicity in modern oncology. This increased importance of dermatologic toxicities to epidermal growth factor receptor (EGFR) inhibitors can be attributed to several factors: 45% to 100% of patients will develop a papulopustular rash; the rash occurs in cosmetically sensitive areas (the face and upper trunk); it is associated with symptoms of pain and pruritus; and superinfections occur in approximately 30% of patients receiving these agents-all of which lead to dose modification by 76% and discontinuation by 32% of oncologists.
The review by LoRusso is a critical update to what could be considered the most significant dermatologic toxicity in modern oncology. This increased importance of dermatologic toxicities to epidermal growth factor receptor (EGFR) inhibitors can be attributed to several factors: 45% to 100% of patients will develop a papulopustular rash; the rash occurs in cosmetically sensitive areas (the face and upper trunk); it is associated with symptoms of pain and pruritus; and superinfections occur in approximately 30% of patients receiving these agents-all of which lead to dose modification by 76% and discontinuation by 32% of oncologists.
Since the approval of the first EGFR inhibitor in 2004, there are now two tyrosine kinase inhibitors (erlotinib [Tarceva], gefitinib [Iressa]) and two monoclonal antibodies (cetuximab [Erbitux], panitumumab [Vectibix]) available in many countries for several indications, including lung, pancreatic, colorectal, and head and neck squamous cell carcinoma. The integration of these agents into protocols for these tumors has occurred swiftly, due to their low associated incidence of hematopoietic toxicities, ease of administration, and positive effects on clinical outcome. Despite these advantages, their use is hampered by dermatologic toxicities that are clearly delineated in the LoRusso review. Evidence-based management guidelines for these reactions have only recently become available.
Complications and Challenges
In her introduction, LoRusso highlights important factors that are currently under investigation in our SERIES (Skin and Eye Reactions to Inhibitors of EGFR and kinaseS) Clinic, an interdisciplinary clinical program for the management of dermatologic and ocular toxicities to cancer therapies. Specifically, we are studying the impact of dermatologic toxicities on quality of life and infections that complicate these toxicities. Preliminary results from our SERIES clinic have demonstrated that up to 30% of patients with dermatologic toxicities develop complications in the form of bacterial, viral, and fungal infections.
LoRusso also notes how the currently used grading system-the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0-is not adequate for grading the papulopustular rash and other EGFR inhibitor–related toxicities. Efforts by the Multinational Association for Supportive Care in Cancer and the upcoming version 4.0 will hopefully overcome deficiencies in the currently used grading system.
The challenges in addressing the various dermatologic toxicities to EGFR inhibitors are exemplified by Figures 1 and 2 in the LoRusso article. In Figure 1, the various toxicities in anatomically distinct areas dictate that antitoxicity interventions should be measured in the various affected sites. In Figure 2, the asynchronous appearance of these untoward events is described, underscoring the importance of evaluating toxicities at the most relevant points in time. Taken together, these figures emphasize that the development of antitoxicity trials with the use of EGFR inhibitors should take into consideration anatomic location as well as time of evaluation and treatment.
Research in Progress
TABLE 1
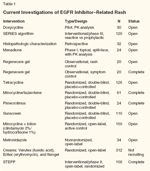
Current Investigations of EGFR Inhibitor-Related Rash
Of the evidence-based treatments for dermatologic toxicities to EGFR inhibitors, rash and pruritus are primary events being investigated (see Table 1). Tetracyclines and minocycline have shown benefit. The number of toxicities being investigated were significantly expanded in the phase II Skin Toxicity Evaluation Protocol with Panitumumab (STEPP) trial, where patients were randomized to either preemptive therapy (the initiation of panitumumab with irinotecan or FOLFIRI [leucovorin, fluorouracil, irinotecan]) or reactive therapy (started when symptoms of dermatologic toxicity first appear). Treatment consisted of doxycycline at 100 mg bid, topical hydrocortisone 1% bid, sunscreen when going outdoors, and moisturizer to cover dry areas. Preliminary results presented at the 10th World Congress on Gastrointestinal Cancer (held in Barcelona, June 25-28, 2008) demonstrate a greater than 50% reduction in grade ≥ 2 dermatologic toxicities of interest when preemptive therapy was instituted.
In summary, LoRusso underscores the need to raise the bar on dermatologic toxicity management, through evidence-based indications. With the increasing use of EGFR inhibitors, it is clear that the results of current trials are a welcome addition to the oncologists’ armamentarium.
Financial Disclosure:Dr. Lacouture has been a consultant for Amgen, OSI, ImClone, and BMS. He has received research funding from OSI and Hana Biosciences and is a speaker for ImClone and BMS.