Adjuvant Chemotherapy for Colon Cancer in the Elderly: Moving From Evidence to Practice
States population will be over 65 years old, with 2% of the population over 84. The corresponding projections for 2050 are 21% and 5%, respectively.[1] These projections underscore the aging of the population, with most recent estimates of life expectancy hitting a record high of 78.1 years.[2] With Americans living longer than ever before, physicians are already seeing larger numbers of elderly patients with cancers whose incidence increases with age, including colon cancer.
ABSTRACT: With a median age at diagnosis of 71 years old and the aging of the US population, colon cancer commonly occurs in the elderly. Adjuvant chemotherapy has been standard of care for stage III disease following complete surgical resection since 1990, but insufficient numbers of patients over 75 years old are participating in clinical trials, and a disparity persists in the administration of standard adjuvant therapy between younger and older Americans despite a meaningful survival advantage for most patients. A large pooled analysis of seven clinical trials supports the administration of adjuvant chemotherapy to otherwise-fit elderly patients, and registry studies have confirmed similar benefits in Medicare beneficiaries. Otherwise-fit elderly patients enrolled in clinical trials do not appear to have more side effects aside from myelosuppression and fatigue. In this review, I discuss the potential benefits and harm of adjuvant therapy in older patients, with a focus on the role of comorbid illness in individualizing decision-making, current standard drug options in the adjuvant setting, and barriers to treatment. Although chronologic age alone should not be an exclusion criterion, more work is needed to establish an optimal and efficient strategy for choosing who would benefit most from adjuvant treatment following surgical resection.
By 2010, 13% of the United States population will be over 65 years old, with 2% of the population over 84. The corresponding projections for 2050 are 21% and 5%, respectively.[1] These projections underscore the aging of the population, with most recent estimates of life expectancy hitting a record high of 78.1 years.[2] With Americans living longer than ever before, physicians are already seeing larger numbers of elderly patients with cancers whose incidence increases with age, including colon cancer.
FIGURE 1
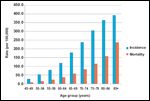
Incidence and Mortality of Colorectal Cancer
American Cancer Society estimates projected that just under 150,000 people would be diagnosed with colorectal cancer, and 50,000 people would die as a result of the disease during 2008.[3] Colon cancer is largely a disease of old age, with a median age at diagnosis of 71 years old and increasing incidence with age (Figure 1). In the 2000–2003 census period, 29% of patients were diagnosed with colon cancer between ages 75 and 84, and 12% at 85 or older. Almost three-quarters of colorectal cancer deaths occur in patients 65 or older.[3,4]
Stage III Colon Cancer: Treatment Disparities
The indication for use of fluorouracil (5-FU)-based adjuvant chemotherapy in regional lymph node–positive (stage III) colon cancer has been well established as standard of care since the publication of a National Institutes of Health consensus statement in 1990,[5] and appears in the National Comprehensive Cancer Network (NCCN) guidelines as a category 1 recommendation (uniform consensus based on high-level evidence).[6] Randomized controlled trials have established that 5-FU–based adjuvant chemotherapy following complete resection of stage III colon cancer provides a relative reduction in subsequent mortality by approximately 30%.[7,8] However, ample evidence exists that decisions about adjuvant therapy after complete resection of colon cancer are often based on age alone.[9-12] Schrag and colleagues reported on this disparity between older and younger patients in a large retrospective cohort study utilizing the Surveillance, Epidemiology and End Results (SEER)/Medicare-linked database during the years 1991–1996. Compared to 78% of patients aged 65 to 69, 74% of those aged 70 to 74, 58% of those aged 75 to 79, and only 34% of those aged 80 to 84 received postoperative chemotherapy within 3 months of surgery. Age remained a strong predictor of chemotherapy after adjustment for potential confounders including severity of noncancer medical illness based on the Charlson/Romano comorbidity index.[12]
Similar results were obtained in a second SEER database study with direct doctor verification of the use of adjuvant therapy, and chart review for comorbidity assessment.[11] A population-based cohort study also reported a lower likelihood of administering adjuvant chemotherapy to older patients with stage III disease in the community (58% in the over-75 age group vs 84% in younger patients), even after adjustment for other prognostic factors.[9]
As to whether there has been any change in practice patterns in the past decade, the evidence is conflicting. A population-based SEER registry study did not show any sign of diminishing treatment disparity according to age over the time period spanning 1997–2002.[13] However, prospective data collection and analysis from over 85,000 patients with stage III colon cancer entered into the National Cancer Data Base-a nationwide oncology outcomes database capturing three-quarters of all newly diagnosed cases of cancer in the United States-showed a clear trend of increasing adjuvant therapy use between 1990–1991, 1995–1996, and 2001–2002 (22%, 26%, and 39% for patients 80 years and older, respectively).[14]
Adjuvant Therapy in the Elderly
Supporting Evidence
The most convincing evidence to date in favor of adjuvant chemotherapy comes from a landmark pooled analysis of seven randomized phase III trials comparing 5-FU–based chemotherapy to no postoperative adjuvant treatment for patients with resected stage II or III colon cancer.[15] The total enrollment cohort was 3,351 patients, 15.1% of whom were over 70 years old. The investigators observed a 7% absolute improvement in 5-year survival attributable to therapy, with no significant interaction between age and treatment effect. Older age had no impact on risk of recurrence but was prognostic for survival, as would be expected given the increased risk of dying from other causes with advancing age. Due to the small proportion (0.7%) of octogenarian participants, the analysis results need to be interpreted with more caution in this age group.
This concern highlights a systemic issue applicable to many published clinical trials. Elderly patient enrollment remains poor even for diseases such as colon cancer that predominantly affect the elderly.[16,17] This is either due to exclusion of elderly patients or very poor accrual of older patients despite the absence of an age cutoff in the exclusion criteria, a phenomenon that tends to be magnified in early adjuvant trials.[18]
While it is clear that selected robust elderly patients with stage III colon cancer who meet clinical trial enrollment criteria derive the same magnitude of benefit from adjuvant therapy as their younger counterparts, an important issue is whether we can extrapolate these benefits to the general population. Two separate observational cohort analyses from the SEER/Medicare population-based registry spanning the mid-1990s corroborated the results of the meta-analysis. Both studies restricted the analysis to Medicare beneficiaries with stage III colon cancer. Sandararajan and colleagues reported a hazard ratio of 0.66 (95% confidence interval [CI] = 0.60–0.73) for the addition of adjuvant therapy.[19] Iwashyna and Lamont reported a corresponding point estimate of 0.73 (95% CI = 0.65–0.82).[20] In both studies, the very elderly were well represented, with one-fifth of patients 80 years or older. In the latter study, an interaction term created for the association between treatment effect and age was not significant.
A third more contemporary analysis found that among patients over 66 years old, increasing age was associated with decreasing survival benefit of chemotherapy, even after adjustment for important prognostic factors. However, the hazard ratio for a 90-year-old patient remained significant at 0.58. It is not clear if the modifying effect of age was adjusted for actual treatment received, a potentially important confounder.[21]
Two recent registry studies reported that one-third of older patients do not complete the full 6 months of adjuvant therapy, and that early treatment discontinuation was associated with a decline in survival.[22,23] Aside from increasing age, factors associated with early discontinuation included having a higher comorbidity score, being female, being widowed or single, and rehospitalization during treatment.
Toxicity and Quality of Life
Elderly patients may not be offered adjuvant therapy as a result of the perception that treatment will be more toxic.[24] Although hospitalization rates for treatment complications rise modestly with increasing age,[12] pooled analyses of adjuvant chemotherapy trials have not reported more side effects in older patients aside from myelosuppression[15,25] and fatigue.[25] Individual studies also reported an increased incidence of mucositis in patients over 70 years old for bolus 5-FU,[26,27] but this did not bear out in the larger joint analyses. For patients enrolled in the MOSAIC trial, which compared the use of adjuvant 5-FU–based chemotherapy with and without oxaliplatin (Eloxatin), researchers found no difference in dose intensity between patients younger than 70 and ≥ 70 years old for either 5-FU or oxaliplatin, but 5% fewer older patients completed all 12 cycles compared with the younger group.[25] The study also excluded patients over 75 years old.[28]
Limited data have been published on quality of life in patients with colorectal cancer. A SEER-based population study reported a high quality of life in individuals whose disease was in remission at or beyond 3 years, regardless of age.[29]
Treatment Barriers for Eligible Elderly Patients
r efficacy and toxicity of adjuvant regimens in fit older patients, why do many elderly patients fail to receive appropriate postoperative adjuvant treatment after having undergone surgery? The answer is likely multifactorial. Possible contributing factors include physical frailty or comorbid disease, lack of social, psychological and financial support, and both physician and patient perceptions regarding treatment efficacy in the elderly and their ability to tolerate systemic chemotherapy.[30]
While aging is invariably associated with a gradual depletion in physiologic reserve, chronologic age is generally an imperfect surrogate for physiologic age. It is the accurate measurement of physiologic age that will enable us to appropriately select patients who can withstand curative but potentially toxic adjuvant treatment. The elderly patient’s priorities must also be considered in the decision process. In a survey, older cancer patients were as likely as their younger counterparts to accept chemotherapy, but having accepted treatment, were less likely to trade quality of life for survival gain.[31] While patients may choose not to receive treatment, they are clearly influenced by the advice of their treating physician in their decision-making.[32]
Risk Stratification: Who Should Receive Chemotherapy?
Elderly patients enrolled in studies make up a highly select fit subset that is not necessarily representative of the larger elderly population for whom treatment decisions are being made in the community. The challenge lies in the application of clinical trial evidence to individual elderly patients, taking into account important considerations including physical frailty, other comorbid illness, risk of complications from treatment, and degree of support available to a given individual.
Of key importance is the presence of comorbid conditions that can impact upon a patient’s physiologic reserve to withstand systemic treatments and can act as a competing cause of mortality.[33] Older patients are more likely to be coping with other major physical illnesses at the time of cancer diagnosis, and the presence of comorbid conditions may be associated with more advanced stage at diagnosis.[34] Although intuitively related, comorbidity burden and functional status assessments were found to provide independent information when administered as part of a battery of diagnostic instruments to elderly cancer patients.[35] The ideal prognostic index will thus factor in comorbidity information and functional status, and would be able to predict mortality risk as well as the impact of cancer treatment.
Screening Tools
In recent years, numerous publications have addressed the applicability and impact of a comprehensive geriatric assessment (CGA) for elderly cancer patients.[36,37] The key domains of any screening tool covered in the CGA include evaluation of an older individual’s functional status, comorbid medical conditions, cognition, nutritional status, psychological state, and a medication review. The multidisciplinary CGA is followed by development of a coordinated intervention plan to optimize the health and autonomy of the individual, and should also aid in the appropriate cancer treatment selection. The CGA can be used to optimize functional status and improve quality of life in cancer patients, but its impact on survival is less clear.[37] Such assessment is resource-intensive and may be necessary only for frail elderly patients with significant physical illnesses.
Shorter instruments that can effectively select patients for more comprehensive assessment exist and are being explored as screening options.[38,39] A core component of any instrument will be a functional evaluation including assessment of activities of daily living (ADL) and instrumental ADL, which can provide important predictive information regarding morbidity and mortality.[40]
TABLE 1

Life Expectancy After Stage III Colorectal Cancer Diagnosis
A recent life-table analysis of older individuals with stage III colon cancer revealed a life expectancy of approximately 5 years for a 67-year-old patient with three or more chronic comorbid illnesses. This compares to 3 years for the corresponding 81 year old (Table 1).[41] With four-fifths of disease recurrences occurring within the first 3 years after definitive treatment,[42] all but the oldest of elderly patients with multiple comorbid illnesses may be considered as potentially benefitting from adjuvant chemotherapy following surgery. Overall survival in the metastatic setting ranges from 6 months without therapy to beyond 24 months with optimal treatment,[43] providing a strong rationale for consideration of adjuvant treatment and discussion with most patients.
TABLE 2

5-yr Overall Survival Estimates With and Without Adjuvant Therapy Derived From Adjavant! Online
The decision to select a patient for adjuvant therapy remains challenging. Web-based calculators such as Adjuvant! Online (www.adjuvantonline.com) can quantify outcomes and predict the degree of benefit from treatment (Table 2). Adjuvant! Online incorporates life expectancy for a given patient’s age and gender, and an estimate of comorbid disease burden. These models can also serve as adjuncts to aid discussions with individual patients with adequate numeracy skills, but do not supersede physician judgment or patient choice.
Treatment Options
The safety and efficacy of specific drugs and regimens have not been studied in large metastatic or adjuvant phase III studies focusing primarily on the elderly. However, numerous phase II studies have been published and recently reviewed.[44] These trials have largely shown comparable outcomes to younger patients in different metastatic settings.
Since adjuvant 5-FU plus levamisole following surgery for stage III colon cancer became standard of care in 1990,[5,7,8] numerous large phase III studies have replaced levamisole with leucovorin[45] and compared less toxic infusional regimens and oral capecitabine (Xeloda) to bolus 5-FU plus leucovorin.[46-48] The most recent development is the addition of oxaliplatin to infusional 5-FU and leucovorin (FOLFOX), with improved overall survival at a median follow-up of 6 years.[28,49] Three of these studies excluded patients over 75 years of age.[28,46,48] Among the three studies reporting descriptive data according to age ranges, 14% to 25% of patients were over 70 years old.[28,45,48] In only a single trial older age itself was associated with worse disease-free and overall survival,[45] and none of the publications reported toxicity data according to age group.
In light of safety data available from pooled analyses[15,25] pointing to a modest increase in the rate of severe hematologic toxicity in the fit elderly accrued to studies, one may choose less toxic options for more frail elderly patients who have significant comorbid disease that may affect their ability to tolerate treatment. These alternatives include 6 months of capecitabine monotherapy or 3 months of infusional 5-FU.[47,48] Given the added risk of significant myelosuppression in the elderly, FOLFOX can also be administered with growth factor support to maintain dose intensity, or if necessary, by omission of the 5-FU bolus.
Another established drug regimen in the metastatic setting is the combination of capecitabine, oxaliplatin, and leucovorin (XELOX), with comparable efficacy and safety in the metastatic setting,[50] including an incremental dosing schedule in elderly patients.[51] The adjuvant XELOXA study comparing XELOX to standard bolus 5-FU regimens has reported safety data showing a tolerable toxicity profile for the combination. However, when the data were analyzed by age, patients over 65 years old in the XELOX arm were more likely to experience diarrhea and dehydration than younger patients.[52] Efficacy data have not yet been published.
The angiogenesis inhibitor bevacizumab (Avastin) and the epidermal growth factor receptor inhibitor cetuximab (Erbitux) are active in the first-line treatment of metastatic disease. They are currently being evaluated in various drug combinations in the National Surgical Adjuvant Breast and Bowel Project (NSABP) C-08 trial, the AVANT (AVastin adjuvANT) trial, the North Central Cancer Treatment Group (NCCTG) N0147 trial and the Pan European Trials in Adjuvant Colon Cancer (PETTAC) 8 trial. With the possible future addition of bevacizumab to current standard adjuvant treatments, extra care in older patient selection will be necessary given the increased risk of arterial thromboembolism in patients over 65 years old and in patients with a prior history of arterial thromboembolic events.[53]
Stage II Colon Cancer

Adjuvant therapy for node-negative or stage II colon cancer has not been shown to improve overall survival,[54,55] although the disease-free survival benefit with the addition of oxaliplatin to infusional 5-FU therapy in a subset of stage II patients with high-risk features is similar in magnitude to that for patients with stage III disease.[49] Current NCCN guidelines include adjuvant chemotherapy as an option for patients with stage II disease, but the panel does not recommend FOLFOX for T3 lesions (ie, where the tumor invades through the muscularis propria into the subserosa or into nonperitonealized pericolic tissues) without high-risk features.[6] These criteria should apply to all patients, regardless of age.
Conclusions
Adjuvant 5-FU–based chemotherapy following surgical resection has been standard of care for stage III colon cancer for 18 years, but fewer patients over 70 years old are receiving it. Pooled data analyses from numerous clinical trials and registries support the view that chronologic age in itself should not be a selection criterion for adjuvant therapy, although trial data in the very elderly (> 80 years old) are more lacking.
For all patients, an assessment of other medical problems, functional status, and anticipated life expectancy is necessary. Otherwise-fit elderly patients should be offered one of the standard adjuvant treatment options with a detailed discussion including potential benefits, treatment risks, and patient choice. Survival benefit may be important for many patients, and toxicity is manageable with attention to the increased risk of myelosuppression in the elderly. Less toxic options can be offered to patients who re more frail.
Financial Disclosure: The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. US Census Bureau: U.S. interim projections by age, sex, race, and Hispanic origin: 2000-2050. Available at www.census.gov. Accessed Jan 18, 2009. Internet-release date: March 18, 2004.
2. Heron MP, Hoyert DL, Xu J, et al: Deaths: Preliminary data for 2006, in National Vital Statistics Reports: Center for Disease Control & Prevention, pp 1-52, 2008.
3. Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2008. CA Cancer J Clin 58:71-96, 2008.
4. Yancik R, Ries LA: Aging and cancer in America. Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am 14:17-23, 2000.
5. NIH consensus conference: Adjuvant therapy for patients with colon and rectal cancer. JAMA 264:1444-1450, 1990.
6. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: Colon cancer, version 4.2008. Available at www.nccn.org. Accessed Jan 18, 2009.
7. Laurie JA, Moertel CG, Fleming TR, et al: Surgical adjuvant therapy of large-bowel carcinoma: An evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol 7:1447-1456,1989.
8. Moertel CG, Fleming TR, Macdonald JS, et al: Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322:352-358, 1990.
9. Kahn KL, Adams EE, Chrischilles DP, et al: Are we use of chemotherapy for elderly stage III colon cancer patients? An analysis from the Cancer Care Outcomes & Research Surveillance Consortium (CanCORS) (abstract 6500). J Clin Oncol 26(15S):337s, 2008.
10. Mahoney T, Kuo YH, Topilow A, et al: Stage III colon cancers: Why adjuvant chemotherapy is not offered to elderly patients. Arch Surg 135:182-185, 2000.
11. Potosky AL, Harlan LC, Kaplan RS, et al: Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol 20:1192-1202, 2002.
12. Schrag D, Cramer LD, Bach PB, et al: Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst 93:850-857, 2001.
13. Davidoff AJ, Choti MA, Zuckerman IH, et al: Is there evidence of diminishing disparities in treatment (tx) with adjuvant (adj) chemotherapy (Ch) among elderly (E) stage 3 colon cancer (CC) patients (pts)? An analysis of 8,374 pts from SEER-Medicare data (abstract 6574). J Clin Oncol 26(15S):355s, 2008.
14. Jessup JM, Stewart A, Greene FL, et al: Adjuvant chemotherapy for stage III colon cancer: Implications of race/ethnicity, age, and differentiation. JAMA 294:2703-2711, 2005.
15. Sargent DJ, Goldberg RM, Jacobson SD, et al: A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 345:1091-1097, 2001.
16. Hutchins LF, Unger JM, Crowley JJ, et al: Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 341:2061-2067, 1999.
17. Sateren WB, Trimble EL, Abrams J, et al: How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol 20:2109-2117, 2002.
18. Lewis JH, Kilgore ML, Goldman DP, et al: Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 21:1383-1389, 2003.
19. Sundararajan V, Mitra N, Jacobson JS, et al: Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med 136:349-357, 2002.
20. Iwashyna TJ, Lamont EB: Effectiveness of adjuvant fluorouracil in clinical practice: A population-based cohort study of elderly patients with stage III colon cancer. J Clin Oncol 20:3992-3998, 2002.
21. Zuckerman IH, Davidoff AJ, Onukwugha E, et al: Effect of age on survival benefit of adjuvant chemotherapy in elderly stage III colon cancer patients: A population-based analysis (abstract 4014). J Clin Oncol 26(15S):181s, 2008.
22. Dobie SA, Baldwin LM, Dominitz JA, et al: Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst 98:610-619, 2006.
23. Neugut AI, Matasar M, Wang X, et al: Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol 24:2368-2375, 2006.
24. Keating NL, Landrum MB, Klabunde CN, et al: Adjuvant chemotherapy for stage III colon cancer: Do physicians agree about the importance of patient age and comorbidity? J Clin Oncol 26:2532-2537, 2008.
25. Goldberg RM, Tabah-Fisch I, Bleiberg H, et al: Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 24:4085-4091, 2006.
26. Popescu RA, Norman A, Ross PJ, et al: Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol 17:2412-2418, 1999.
27. Zalcberg J, Kerr D, Seymour L, et al: Haematological and non-haematological toxicity after 5-fluorouracil and leucovorin in patients with advanced colorectal cancer is significantly associated with gender, increasing age and cycle number. Tomudex International Study Group. Eur J Cancer 34:1871-1875, 1998.
28. Andre T, Boni C, Mounedji-Boudiaf L, et al: Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343-2351, 2004.
29. Ramsey SD, Andersen MR, Etzioni R, et al: Quality of life in survivors of colorectal carcinoma. Cancer 88:1294-1303, 2000.
30. Muss HB, Cohen HJ, Lichtman SM: Clinical research in the older cancer patient. Hematol Oncol Clin North Am 14:283-291, 2000.
31. Yellen SB, Cella DF, Leslie WT: Age and clinical decision making in oncology patients. J Natl Cancer Inst 86:1766-1770, 1994.
32. Newcomb PA, Carbone PP: Cancer treatment and age: Patient perspectives. J Natl Cancer Inst 85:1580-1584, 1993.
33. Yancik R, Wesley MN, Ries LA, et al: Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: A population-based study. Cancer 82:2123-2134, 1998.
34. Zafar Y, Provenzale D, Herndon JE II, et al: The impact of comorbidity on diagnosis and treatment of colorectal cancer (CRC) (abstract 261). Proceedings of the 2007 Gastrointestinal Cancers Symposium. Orlando, Fla; Jan 19-21, 2007.
35. Extermann M, Overcash J, Lyman GH, et al: Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 16:1582-1587, 1998.
36. Extermann M, Hurria A: Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 25:1824-1831, 2007.
37. Maas HA, Janssen-Heijnen ML, Olde Rikkert MG, et al: Comprehensive geriatric assessment and its clinical impact in oncology. Eur J Cancer 43:2161-2169, 2007.
38. Hurria A, Gupta S, Zauderer M, et al: Developing a cancer-specific geriatric assessment: A feasibility study. Cancer 104:1998-2005, 2005.
39. Rodin MB, Mohile SG: A practical approach to geriatric assessment in oncology. J Clin Oncol 25:1936-1944, 2007.
40. Repetto L, Fratino L, Audisio RA, et al: Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology study. J Clin Oncol 20:494-502, 2002.
41. Gross CP, McAvay GJ, Krumholz HM, et al: The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: Implications for screening. Ann Intern Med 145:646-653, 2006.
42. Sargent DJ, Wieand HS, Haller DG, et al: Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 23:8664-8670, 2005.
43. Kelly H, Goldberg RM: Systemic therapy for metastatic colorectal cancer: Current options, current evidence. J Clin Oncol 23:4553-4560, 2005.
44. Sanoff HK, Bleiberg H, Goldberg RM: Managing older patients with colorectal cancer. J Clin Oncol 25:1891-1897, 2007.
45. Haller DG, Catalano PJ, Macdonald JS, et al: Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of Intergroup 0089. J Clin Oncol 23:8671-8678, 2005.
46. Andre T, Colin P, Louvet C, et al: Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: Results of a randomized trial. J Clin Oncol 21:2896-2903, 2003.
47. Chau I, Norman AR, Cunningham D, et al: A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol 16:549-557, 2005.
48. Twelves C, Wong A, Nowacki MP, et al: Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 352:2696-2704, 2005.
49. de Gramont A, Boni C, Navarro M, et al: Oxaliplatin/5FU/LV in adjuvant colon cancer: Updated efficacy results of the MOSAIC trial, including survival, with a median follow-up of six years (abstract 4007). J Clin Oncol 25(18S):165s, 2007.
50. Cassidy J, Tabernero J, Twelves C, et al: XELOX (capecitabine plus oxaliplatin): Active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol 22:2084-2091, 2004.
51. Comella P, Natale D, Farris A, et al: Capecitabine plus oxaliplatin for the first-line treatment of elderly patients with metastatic colorectal carcinoma: Final results of the Southern Italy Cooperative Oncology Group Trial 0108. Cancer 104:282-289, 2005.
52. Schmoll HJ, Cartwright T, Tabernero J, et al: Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: A planned safety analysis in 1,864 patients. J Clin Oncol 25:102-109, 2007.
53. Scappaticci FA, Skillings JR, Holden SN, et al: Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 99:1232-1239, 2007.
54. Gill S, Loprinzi CL, Sargent DJ, et al: Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J Clin Oncol 22:1797-1806, 2004.
55. Schrag D, Rifas-Shiman S, Saltz L, et al: Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol 20:3999-4005, 2002.