Toward Evidence-Based Management of the Dermatologic Effects of EGFR Inhibitors
Recent results from prospective, controlled trials, specifically evaluating strategies for preventing or reducing the severity of the dermatologic effects of EGFR inhibitors, represent the first step toward an evidence-based approach to the prevention and management of these important effects.
ABSTRACT: Agents that inhibit epidermal growth factor receptor (EGFR) signaling are associated with dermatologic effects, primarily a papulopustular rash. These effects are generally mild-to-moderate in severity, but may negatively affect quality of life and lead to treatment delays, dose modifications, and discontinuation of therapy. These findings underscore the need for effective ways to prevent and manage the dermatologic effects of EGFR inhibitors. Evidence-based guidelines are lacking, but algorithms have been developed based on the available evidence, clinical experience, and our understanding of the effects of EGFR inhibition in skin. More recently, the first results from prospective, controlled trials specifically evaluating strategies for preventing or reducing the severity of cutaneous reactions have been reported. These reports represent the first step toward an evidence-based approach to the prevention and management of these important effects.
The epidermal growth factor receptor (EGFR) is a validated target for cancer therapy, and treatment with EGFR inhibitors (EGFRIs) is an effective antitumor strategy associated with fewer adverse events than conventional chemotherapy, with a distinct toxicity profile.[1,2] The most common toxicity associated with EGFRI therapy is dermatologic reactions, particularly a papulopustular rash, which occurs in nearly all patients.[3] Given the important role of EGFR in maintaining skin integrity, these effects are not unexpected. Cutaneous toxicity has been observed with all agents that target EGFR, and is therefore considered a class effect.
EGFRI-related dermatologic effects are generally mild or moderate in severity. Even when they are not severe, these events may pose clinical concerns, such as risk of secondary infections, and patients must find ways to cope with chronic discomfort, itching, and the appearance of the rash.[4] The rash predominantly affects visible areas of the body, which can cause distress and anxiety in some patients and negatively affect self-image and self-esteem. This may increase the likelihood of poor compliance or discontinuing therapy, especially if being considered in the future for use in the adjuvant setting.[5]
Recent studies have formally assessed the impact of EGFRI-related rash on quality of life and have noted a direct correlation between National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) grade of the rash and the dermatology-specific quality of life. Investigators have noted that of the three domains evaluated in a patient population that developed a papulopustular rash while being treated with EGFR inhibitors-symptomatic, functional, and emotional-the domain that was impacted the most was emotional.[6,7] Rash can lead to treatment discontinuation in up to one-third of patients treated with EGFRIs.[1]
The potential for negative clinical consequences and the risk of impaired quality of life and poor treatment compliance underscores the need for effective management strategies for EGFRI-related dermatologic reactions. Current approaches to rash management are empirical and vary widely among care providers.[1] Evidence-based guidelines have been impossible to establish due to the lack of data from adequately controlled clinical trials.[6,8] Consensus reports regarding the optimal approach to rash management have been developed, but these are based largely on anecdotal evidence and clinical experience.[4,9-11] Prospective, controlled trials evaluating strategies for managing or preventing EGFRI-related rash are underway, and the first results from some of these trials were recently reported.[12,13] These trials represent the first steps beyond current strategies toward an evidence-based approach to the management of EGFRI-related rash.
Clinical Manifestations
FIGURE 1
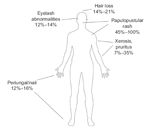
Distribution and Incidence of Dermatologic Effects of EGFRI Therapy
The dermatologic effects of EGFRIs are unique in the spectrum of drug-induced skin changes.[11] Papulopustular rash is the most common manifestation, but other cutaneous effects, such as xerosis, pruritus, paronychia, and changes in hair growth, have been observed (Figure 1).[6]
Papulopustular Rash
Prospective studies specifically evaluating the cutaneous effects of EGFRIs describe the rash as pruritic, follicular, papulopustular, erythematous lesions.[14,15] The most commonly affected area is the face, including the nose, chin, nasolabial folds, perioral area, cheeks, and forehead. The shoulders and trunk may also be affected, particularly the V-shaped areas of the chest and back.[11,16] In some cases, the lower back, abdomen, and limbs may also be affected.[11]
Severe cases of rash are relatively uncommon; in patients treated with cetuximab (Erbitux), for example, the reported incidence of grade 3/4 rash is 5% to 17%.[17,18] The reported incidence of rash with gefitinib (Iressa) (25%–33%) is somewhat lower than that reported with erlotinib (Tarceva), panitumumab (Vectibix), and cetuximab (75%–90%).[1]
Importantly, EGFRI-related rash does not share the clinical or histologic features of acne vulgaris. Unlike acne, EGFRI-induced rash often produces pruritic lesions, responds to anti-inflammatory medication, fails to respond to acne medications, affects more areas of the body (such as the arms and lower legs), and does not produce comedones.[4,6] Microbiologic cultures are often negative,[14,15] although secondary infections may occur.[4] The papulopustular stage is typically followed by a crusting stage, in which purulent material dries and forms visible crusts on the skin surface. The crusts consist of neutrophilic and keratinocytic debris, parakeratotic cells, fibrin, and serum and should not be confused with an opportunistic infection.[4,6] EGFRI-induced rash should also be differentiated from allergic reactions and steroid-induced acne.[4]
Xerosis and Pruritus
Approximately 7% to 35% of patients treated with EGFRIs develop dry, scaly, or itchy skin (xerosis).[3] Xerosis often affects the limbs and areas previously affected by papulopustular rash.[11,19] It may develop into chronic eczema and increase the risk of secondary infections.[11]
Paronychia/Periungual Inflammation
Paronychia with associated swelling of nail folds has been reported.[20] It occurs in approximately 12% to 16% of patients and is considered a late event, often arising 4 to 8 weeks after treatment initiation.[3,11] Paronychia is characterized by inflammation of the nail fold, painful fissuring, and brittle nails.[11] Severe complications, such as pyogenic granuloma, have been reported.[14]
Changes in Hair Growth/Ocular Toxicity
Treatment with EGFRIs can cause variable effects on hair growth, depending on the location on the body. Hair loss occurs in 14% to 21% of patients, whereas increased growth of eyelashes and eyebrows (trichomegaly) occurs in 12% to 14% of patients.[3,21] Other reported hair changes include development of curlier, finer, or more brittle hair on the scalp, reduced beard growth, and excessive eyelash curling.[7,21]
In particular, trichomegaly of the eyelashes, which can scratch the eye, may be accompanied by conjunctivitis and other ocular disorders that can lead to significant discomfort and blurred vision.[3]
Telangiectasias and Hyperpigmentation
Telangiectasias have been observed and may be related to the indirect antiangiogenic effects associated with EGFR inhibition. If left untreated, telangiectasias will gradually disappear over months, but pulsed dye laser therapy may accelerate this process. Hyperpigmentation appears to be a residual effect of inflammation and usually fades spontaneously over time.[11] Patients should be advised to avoid sun exposure and to use adequate sunscreen to limit exposure to ultraviolet (UV) radiation; this may help avoid hyperpigmentation.[22]
Duration and Onset of Rash
FIGURE 2

Duration and Onset of Rash
The dermatologic effects of EGFR therapy can be generally classified as early effects (sensory disturbances, erythema, edema, papulopustular rash, crusting) and late effects (hyperpigmentation, hair changes, paronychia/fissuring, xerosis) (Figure 2).[6] In most cases, papulopustular rash develops within the first 3 weeks of treatment, and typically peaks in severity 3 to 5 weeks after treatment initiation.[10,18] Delayed onset, ie, after the first 3 weeks of treatment, has been observed in a minority (11%) of patients treated with cetuximab.[18] The severity of the rash may wax or wane during treatment, and spontaneous resolution has been reported, which may complicate assessment of the efficacy of interventions.[16] After treatment is stopped, the rash usually resolves within 4 weeks but may leave residual hyperpigmentation and xerosis.[10,19]
Paronychia and fissuring are late effects that usually develop 2 to 4 months after treatment initiation. These effects can be persistent and endure for several months after treatment cessation. Hair changes have a variable onset; they can sometimes occur as early as 7 to 10 weeks after treatment initiation, or up to several months later. Xerosis develops after the papulopustular rash has resolved, which usually occurs during the second month of treatment.[10]
Mechanisms
EGFR is expressed in undifferentiated and proliferating keratinocytes in the basal and suprabasal layers of the epidermis.[6] Keratinocytes rely on EGFR to regulate proliferation, differentiation, migration, and survival.[6,23] EGFR expression is particularly abundant in and around follicles. Normally, activated EGFR and several EGFR-dependent downstream factors, such as MAPK and Ki67, can be found in keratinocytes in the basal layer of the epidermis.[6]
The effects of EGFRIs on skin are directly linked to their ability to interfere with EGFR signaling.[11,15] Treatment with an EGFRI abolishes EGFR expression in basal layer keratinocytes, and increases expression of factors related to premature differentiation and growth arrest, such as p27KIP1, KRT1, and STAT3. In fact, inhibition of EGFR signaling has been shown to reduce DNA synthesis, arrest growth, and induce premature differentiation in keratinocytes. It also inhibits migration and promotes adhesion, which prevents the normal excursion of keratinocytes from the basal layer to the corneal layer, which is necessary for their maturation. In addition, inhibition of EGFR attracts inflammatory cells to the skin, causing apoptosis and tissue damage. As a result of these changes, the epidermis and corneal layer become thinner, which impairs barrier function and sensitizes the skin to UV radiation.[6]
FIGURE 3
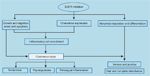
EGFRI Mechanisms and Dermatologic Side Effects
The cascade of molecular and cellular events initiated by EGFR inhibition in skin corresponds with many of the specific clinical manifestations seen during EGFRI therapy (Figure 3). Immediate symptoms of burning, redness, and swelling, for example, are linked to the inhibition of EGFR in basal keratinocytes, which attracts inflammatory cells and causes vascular dilation and increased permeability, all of which activate nociceptive fibers in the skin. Delayed effects of abnormal keratinocyte maturation and differentiation are expressed as late clinical effects, such as xerosis and hair and nail changes.[6]
Interventions
Controlled Clinical Trials
FIGURE 4

Clinical Trial of Strategy to Manage EGFRI-Induced Rash
One of the first randomized controlled clinical trials to prospectively evaluate strategies for managing EGFRI-induced rash was reported by Scope et al. These investigators randomized 48 patients to rash prophylaxis with an oral semisynthetic tetracycline (minocycline, 100 mg daily) or placebo for 8 weeks, beginning on the same day as the initiation of cetuximab therapy. Patients also received topical tazarotene 0.05% (Tazorac), twice daily on one side of the face. Overall, the number of lesions increased rapidly during the first 4 weeks of therapy and then stabilized by week 8. Patients treated with minocycline had a significantly lower lesion count than those in the placebo group after 4 weeks. The difference between treatment arms was apparent as early as week 1, peaked at weeks 2 and 4, and tapered by the end of the study (Figure 4). Tazarotene appeared to have no significant effect on rash, and a substantial number of patients discontinued tazarotene due to irritation or lack of efficacy.[13]
The investigators concluded that minocycline prophylaxis effectively reduced the severity of rash during the first month of cetuximab therapy. The protective effects were diminished by the end of the second month of therapy, suggesting that a 2-month course of minocycline, beginning at the same time as initiation of cetuximab therapy, warrants further investigation.[13]
Similar results were reported in a second placebo-controlled trial conducted by the North Central Cancer Treatment Group (NCCTG). In this phase III trial, 61 patients who had begun treatment with an EGFRI and had not yet developed rash in the 7 days prior to study entry, were randomized to oral tetracycline (500 mg twice daily) or placebo for 4 weeks. There was no difference in rash incidence at 4 weeks, but there was a significant reduction in rash severity favoring tetracycline. Tetracycline was also associated with improvements in itching, according to Skindex-16 scores. Thus, tetracycline appeared to reduce the severity of rash and improve some measures of quality of life in patients treated with EGFRIs.[12]
Anecdotal Evidence
TABLE 1

Management of EGFRI-Induced Dermatologic Effects
Several specific interventions have been reported to help minimize rash and other dermatologic effects (Table 1).[4,6,11,19,20,22,24-26] These interventions are primarily based on anecdotal evidence. General recommendations for patients on the use of makeup, cleansers, emollients, sunscreen, and analgesics have also been formulated.[4] No strategy has yet emerged as a clearly optimal way to prevent or manage EGFRI-related dermatologic effects in the majority of patients. However, our improved understanding of the effects of EGFR inhibition in the skin and how they correlate with clinical manifestations is helping to develop a more rational approach to selecting an appropriate intervention. In addition to these interventions, dose modifications or discontinuation of EGFRI therapy may be considered, particularly in cases of severe rash (Table 2).[27-29]
TABLE 2

Recommended Dose Modifications for EGFRI-Induced Rash
Given the lack of evidence-based guidelines, it is not surprising that approaches used in clinical practice to manage EGFRI-associated dermatologic effects vary widely, as was found in a recently reported survey of US clinicians involved in administering EGFRI therapy. Providers who used topical or topical and oral treatment in combination had significantly more patients in whom rash intervention was felt to have made a difference, compared with those who provided oral treatment only-a finding that may provide the basis for a clinical trial comparing combined topical and oral therapy with either therapy alone. Notably, the survey also revealed that rash led to discontinuation of EGFRI therapy in 32% of patients. Treatment delays (usually 2–3 weeks) due to rash occurred in 76% of patients. These results highlight the need for effective interventions for EGFRI-induced rash to prevent unnecessary dose interruptions or treatment discontinuation.[1]
Novel Approaches
FIGURE 5

Consensus Guidelines
Currently, the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 is the most commonly used grading scale for rash and other dermatologic effects seen with EGFRIs.[30] These criteria, however, do not account for functional and aesthetic impairment caused by EGFRI-related dermatologic effects.[1] Better grading scales are needed that account for the impact of EGFRI-related rash on the patient’s daily life.[8] The NCI is currently working on a CTCAE version 4.0. It is anticipated that this version will take into consideration the newly observed dermatologic toxicities that result from the EGFRIs and other classes of agents.
Some groups have proposed alternative grading scales that are more specific to EGFRI therapy. Prez-Soler et al have recommended modifying the NCI CTCAE to better reflect the psychosocial impact of rash.[4] For example, they suggest subdividing grade 2 rash according to whether the rash affects the patient’s daily life. Lynch et al have developed new definitions specifically for EGFRI-related rash (mild, moderate, or severe), and devised an algorithm for managing each level of severity (Figure 5).[10]
FIGURE 6
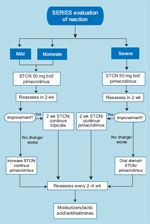
SERIES Guidelines
• SERIES Clinic-Lacouture et al have established the SERIES (Skin and Eye Reactions to Inhibitors of EGFR and kinaseS) Clinic and Cancer Skin Program, an interdisciplinary clinic designed to effectively manage EGFRI-induced rash. The SERIES clinic focuses on effective collaboration and early intervention to provide prompt and efficient care and avoid unnecessary treatment discontinuations, delays, or dose reductions. The clinic is composed of dermatologists, ophthalmologists, and basic researchers and ensures timely access to relevant specialists through better coordination between oncology and dermatology offices. Scheduling of patients treated with EGFRIs is prioritized so that they may be seen by a dermatologist in a timely manner. An algorithm was devised to standardize the management of patients who develop cutaneous effects (Figure 6). Initial evaluation and care is the responsibility of a dermatologist, who refers patients to other subspecialists as needed.
The clinic also provides long-term follow-up and care, as dermatologic effects can develop at any time during the course of therapy. Patients are provided written and verbal information on how to recognize the dermatologic effects of EGFRI therapy and the importance of follow-up. The authors report that the program has been well received by patients, oncologists, and allied health staff, who appreciate the efficiency, improved communication, and proactive/preventive approach.[3]
FIGURE 7
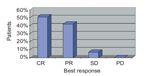
SERIES Study Results
The efficacy of the intervention used in the SERIES clinic, which involves a combination of oral tetracyclines, topical calcineurin inhibitors, and oral corticosteroids, was recently evaluated in a retrospective analysis of 45 patients treated with erlotinib or cetuximab.[31] The SERIES approach reduced the severity of rash independently of the type of EGFRI used. Best response to intervention is shown in Figure 7. In most patients, rash severity improved by 1 to 2 grades. In patients treated with erlotinib, 8% required dose reductions due to rash and no patients stopped erlotinib therapy due to rash. In patients treated with cetuximab, 22% required dose modifications due to rash, and 17% stopped treatment due to rash.
These findings are consistent with the results of the practitioner survey that showed dermatologic toxicities having a notable impact on treatment delivery[1]-an impact that is greater than what would be expected from the reported clinical trial experience (rates of discontinuation are less than 10%).[17,32,33] This gap in the optimization of management in daily practice underscores the need for earlier, more proactive approaches. Trials evaluating prophylaxis for cetuximab-induced rash are underway.[31]
Conclusions
The clinical impact of EGFRI-associated dermatologic effects and their potential effect on treatment compliance and quality of life has not been fully defined, but is likely to be considerable. Current consensus emphasizes the importance of developing an interdisciplinary approach involving specialists in oncology and dermatology. Treatment algorithms and recommendations have been developed to help clinicians prevent or reduce these effects. These approaches are based on the available clinical evidence and an improved understanding of how inhibition of EGFR affects the skin.
Emerging data from controlled clinical trials will help determine the optimal approach, and additional trials evaluating strategies for both the prevention and management of dermatologic effects are underway. These trials, together with coordinated efforts to formulate consensus management guidelines, will help establish a more objective, evidence-based approach to the management of EGFRI-related dermatologic effects.
Financial Disclosure:Dr. LoRusso has received consulting fees or other honoraria from Abraxis, BMS, Genentech, GSK, NCI, Novartis, Sanofi-Aventis, Syndax, and Takeda. She has also served on the scientific advisory boards for Abraxis, AstraZeneca, BMS, Eisai, Genentech, GSK, Novartis, Pfizer, Sanofi-Aventis, and Takeda, a scientific review committee for NCI, a data safety monitoring committee for Syndax, and has been a speaker for Genentech, GSK, and Sanofi-Aventis.
References:
1. Boone SL, Rademaker A, Liu D, et al: Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: Survey results. Oncology 72:152-159, 2007.
2. Dancey J, Sausville EA: Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev Drug Discov 2:296-313, 2003.
3. Lacouture ME, Basti S, Patel J, et al: The SERIES clinic: An interdisciplinary approach to the management of toxicities of EGFR inhibitors. J Support Oncol 4:236-238, 2006.
4. Pérez-Soler R, Delord JP, Halpern A, et al: HER1/EGFR inhibitor-associated rash: Future directions for management and investigation outcomes from the HER1/EGFR Inhibitor Rash Management Forum. Oncologist 10:345-356, 2005.
5. Barankin B, DeKoven J: Psychosocial effect of common skin diseases. Can Fam Physician 48:712-716, 2002.
6. Lacouture ME: Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 6:803-812, 2006.
7. Witherspoon JN, Wagner L, Rademaker A, et al: Correlation of patient characteristics and NCI-Common Terminology Criteria for Adverse Events (CTCAE) v 3.0 grading with dermatology-related quality of life (QoL) in patients with EGFR inhibitor-induced rash (abstract 9559). J Clin Oncol 26(15S):516s, 2008.
8. Agero ALC, Dusza SW, Benvenuto-Andrade C, et al: Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 55:657-670, 2006.
9. Bernier J, Bonner J, Vermorken JB, et al: Consensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann Oncol 19:142-149, 2008.
10. Lynch TJ, Kim ES, Eaby B, et al: Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: An evolving paradigm in clinical management. Oncologist 12:610-621, 2007.
11. Segaert S, Van Cutsem E: Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol 16:1425-1433, 2005.
12. Jatoi A, Rowland K, Sloan JA, et al: Does tetracycline prevent/palliate epidermal growth factor receptor (EGFR) inhibitor-induced rash? A phase III trial from the North Central Cancer Treatment Group (N03CB) (abstract LBA9006). J Clin Oncol 25(18S):494s, 2007.
13. Scope A, Agero ALC, Dusza SW, et al: Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol 25:5390-5396, 2007.
14. Roé E, Garcia Muret MP, Marcuello E, et al: Description and management of cutaneous side effects during cetuximab and erlotinib treatments: a prospective study of 30 patients. J Am Acad Dermatol 55:429-437, 2006.
15. Busam KJ, Capodieci P, Motzer R, et al: Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol 144:1169-1176, 2001.
16. Jacot W, Bessis D, Jorda E, et al: Acneform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol 151:232-257, 2004.
17. Bonner JA, Harari PM, Giralt J, et al: Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567-578, 2006.
18. Cunningham D, Humblet Y, Siena S, et al: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337-345, 2004.
19. Kimyai-Asadi A, Jih MH: Follicular toxic effects of chimeric anti-epidermal growth factor receptor antibody cetuximab used to treat human solid tumours. Arch Dermatol 138:129-131, 2002.
20. Monti M, Mancini LL, Ferrari B, et al: Cutaneous toxicity induced by cetuximab. J Clin Oncol 21:4651-4653, 2003.
21. Dueland S, Sauer T, Lund-Johansen F, et al: Epidermal growth factor receptor inhibition induces trichomegaly. Acta Oncol 42:345-346, 2003.
22. Luu M, Lai SE, Patel J, et al: Photosensitive rash due to the epidermal growth factor receptor inhibitor erlotinib. Photodermatol Photoimmunol Photomed 23:42-45, 2007.
23. Yarden Y, Sliwkowski MX: Untangling the ErbB signalling network. Nat Rev Molec Cell Biol 2:127-137, 2001.
24. Alexandrescu DT, Vaillant JG, Dasanu CA: Effect of treatment with a colloidal oatmeal lotion on the acneform eruption induced by epidermal growth factor receptor and multiple tyrosine-kinase inhibitors. Clin Exp Dermatol 32:71-74, 2007.
25. Mitchell E, Lacouture M, Shearer H, et al: A phase II, open-label trial of skin toxicity evaluation (STEPP in metastatic colorectal cancer patients receiving panitumumab + FOLFIRI or irinotecan-only chemotherapy as 2nd-line treatment (abstract O-021). Presented at the 10th World Congress on Gastrointestinal Cancer; Barcelona, Spain; June 26, 2008.
26. Porzio G, Aielli F, Verna L, et al: Efficacy of pregabalin in the management of cetuximab-related itch. J Pain Symptom Manage 32:397-398, 2006.
27. Erbitux® (cetuximab) [package insert]. Branchburg, NJ: ImClone Systems Incorporated and Princeton, NJ; Bristol-Myers Squibb Company; November 2007.
28. Iressa® (gefitinib tablets) [package insert]. Wilmington, Del; AstraZeneca Pharmaceutical LP; May 2005.
29. Tarceva® (erlotinib tablets) [package insert]. Melville, NY, OSI Pharmaceuticals Inc and South San Francisco, Genentech Inc; May 2007.
30. National Cancer Institute: Common Terminology Criteria for Adverse Events, version 3.0. Available at http://ctep.cancer.gov/reporting/ctc_v30.html. Accessed September 10, 2007.
31. Martin NV, Pacifico V, Lai SE, et al: Management of rash to erlotinib (E) and cetuximab (C): Results from the SERIES (Skin and Eye Reactions to Inhibitors of EGFR and kinaseS) clinic algorithm (abstract 19556). J Clin Oncol 25(18S):711s, 2007.
32. Sobrero A, Maurel J, Fehrenbacher L, et al: EPIC: Phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26:2311-2319, 2008.
33. Van Cutsem E, Nowacki M, Lang I, et al: Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): The CRYSTAL trial (abstract 4000). J Clin Oncol 25(18S):164s, 2007.