(S018) Early Tumor Perfusion Changes Predict Time to Progression in Patients With Recurrent Low-Grade Gliomas Treated With Everolimus Under a Phase II Clinical Trial
Low-grade gliomas (LGGs) are slow-growing, primary brain tumors that frequently recur after primary surgical treatment. Recent work has established the activation of the PI3K/mTOR pathway in most LGGs, raising the possibility that mTOR inhibitors, such as everolimus (RAD001), may benefit patients with LGG. Early imaging markers of treatment response and disease progression are needed to assess patients undergoing investigational therapy.
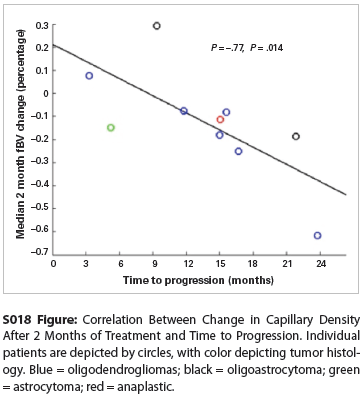
S018 Figure
Michael Wahl, MD, Janine M. Lupo, PhD, Sarah J. Nelson, PhD, Daphne Haas-Kogan, MD; University of California, San Francisco
Purpose: Low-grade gliomas (LGGs) are slow-growing, primary brain tumors that frequently recur after primary surgical treatment. Recent work has established the activation of the PI3K/mTOR pathway in most LGGs, raising the possibility that mTOR inhibitors, such as everolimus (RAD001), may benefit patients with LGG. Early imaging markers of treatment response and disease progression are needed to assess patients undergoing investigational therapy.
Methods: In this phase II clinical trial, 26 patients with recurrent LGG treated with everolimus underwent serial multimodal magnetic resonance imaging every 2 months during treatment. At each time point, the volume of hyperintensity on T2-weighted imaging was manually defined. Maps of imaging parameters were generated, including the fractional blood volume (fBV) and vascular permeability (Kps) from dynamic contrast-enhanced perfusion-weighted imaging, the apparent diffusion coefficient (ADC) and fractional anisotropy (FA) from diffusion-weighted imaging, and MR spectroscopic imaging-derived parameters (peak heights of choline, lipid, and lactate and the choline-to-N-acetylaspartate index, [CNI]). Each parameter was normalized to its median value in normal-appearing white matter, and the median, 10th percentile, and 90th percentile values were computed within the tumor volume, defined by T2 hyperintensity.
Results: At the time of analysis, 11 patients had experienced disease progression (range: 20 d–23.8 mo), 12 had stable disease (median follow-up 18.1 mo), and 3 dropped out of the study due to adverse side effects. Eighteen patients reached the primary study endpoint of freedom from progression at 6 months. Compared with baseline imaging parameters, patients who did not progress in the first 6 months demonstrated a significant decrease in median fBV values within the tumor volume, with a decrease of 17.5% at 4 months and 22.0% at 6 months (P = .010 and .011, respectively, by Wilcoxon signed-rank test). Similarly, these patients demonstrated a decrease in median Kps values of 14.8% at 4 months and 15.7% at 6 months (P = .041 and .070, respectively). Tumor volume, diffusion, and spectroscopic characteristics were not significantly changed during treatment.
Based on these findings, we performed a hypothesis-driven analysis of the relationship between early tumor perfusion changes and time to progression for the 11 patients who experienced disease progression. The change in median fBV at the earliest assessed time point, 2 months after starting treatment, was highly correlated with time to progression (Figure; Spearman’s Ï = -0.77; P = .014). Baseline imaging characteristics were not correlated with progression time.
Discussion: The finding of significantly decreased tumor perfusion characteristics in patients under treatment with everolimus for recurrent LGG is consistent with the known antiangiogenic properties of mTOR inhibitors. Furthermore, we observe a strong correlation between these early tumor perfusion changes and time to progression. This information could thus be used as an early radiographic marker for response to this targeted molecular therapy, potentially identifying patients who would most benefit from treatment.
Proceedings of the 96th Annual Meeting of the American Radium Society - americanradiumsociety.org