3 Things You Should Know About Frontline TROP2-Targeting Antibody-Drug Conjugate/Immune Checkpoint Inhibitor Combinations to Treat Advanced Triple-Negative Breast Cancer
Explore the latest advancements in antibody-drug conjugates for treating metastatic triple-negative breast cancer, enhancing patient outcomes and safety.
The experts.

LEARNING OBJECTIVES
Upon successful completion of this activity, you should be better prepared to:
- Describe the mechanistic rationale for utilizing antibody-drug conjugates (ADCs) to target TROP2 in the management of patients with metastatic triple-negative breast cancer (TNBC)
- Develop evidence-based strategies for integrating ADCs in the management of patients with metastatic TNBC
- Apply effective methods to mitigate adverse events associated with ADCs in patients with metastatic TNBC
- Evaluate recent evidence supporting the evolving role of ADCs in the management of patients with metastatic TNBC
RELEASE DATE: October 1, 2025
EXPIRATION DATE: October 1, 2026
Accreditation/Credit Designation
Physicians’ Education Resource®, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physicians’ Education Resource®, LLC designates this enduring material for a maximum of 0.25 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Acknowledgement of Commercial Support
This activity is supported by an educational grant from Gilead Sciences, Inc.
Off-Label Disclosure and Disclaimer
This activity may or may not discuss investigational, unapproved, or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a health care professional relative to diagnostic, treatment, or management options for a specific patient’s medical condition. The opinions expressed in the content are solely those of the individual faculty members and do not reflect those of PER® or any company that provided commercial support for this activity.
INSTRUCTIONS FOR PARTICIPATION and HOW TO RECEIVE CREDIT
1. Read this activity in its entirety.
2. Go to https://gotoper.com/tnbcresource25trop2adcs-postref to access and complete the posttest.
3. Answer the evaluation questions.
4. Request credit using the drop-down menu.
YOU MAY IMMEDIATELY DOWNLOAD YOUR CERTIFICATE.
Approximately 10% to 15% of all breast cancers are triple negative (TNBC) and are associated with worse survival outcomes compared with hormone receptor–positive and HER2- positive subtypes.1,2TNBC disproportionately affects women who are Black, younger than 40 years, or have a BRCA1 mutation.1 The 5-year relative survival rate for patients with metastatic TNBC is just 15%,1 and chemotherapy provides low objective response rates (ORRs) with short durations of response (DORs) and limited progression-free survival (PFS).3 More recently, the addition of the immune checkpoint inhibitor (ICI) pembrolizumab to chemotherapy has improved the efficacy of first-line treatment for advanced TNBC with a combined positive score (CPS) of greater than or equal to 10.4-6 In the second line, the antibody-drug conjugates (ADCs) sacituzumab govitecan (SG) and fam-trastuzumab deruxtecan have improved efficacy for some patients with advanced TNBC.7-11 In an analysis of 85 samples, high TROP2 expression was most common in TNBC (93%) compared with other breast cancer subtypes (HER2-positive samples, 74%; estrogen receptor–positive samples, 50%), making this protein a rational ADC target for patients with TNBC.12 Combining ADCs and ICIs leads to synergistic tumor killing,13 which may move this combination into the front line for patients with TNBC. Here are 3 things you should know about frontline TROP2-targeting ADC/ICI combinations to treat advanced TNBC.
1/ Frontline SG plus pembrolizumab significantly improves PFS compared with chemotherapy plus pembrolizumab for patients with advanced, PD-L1–positive TNBC.
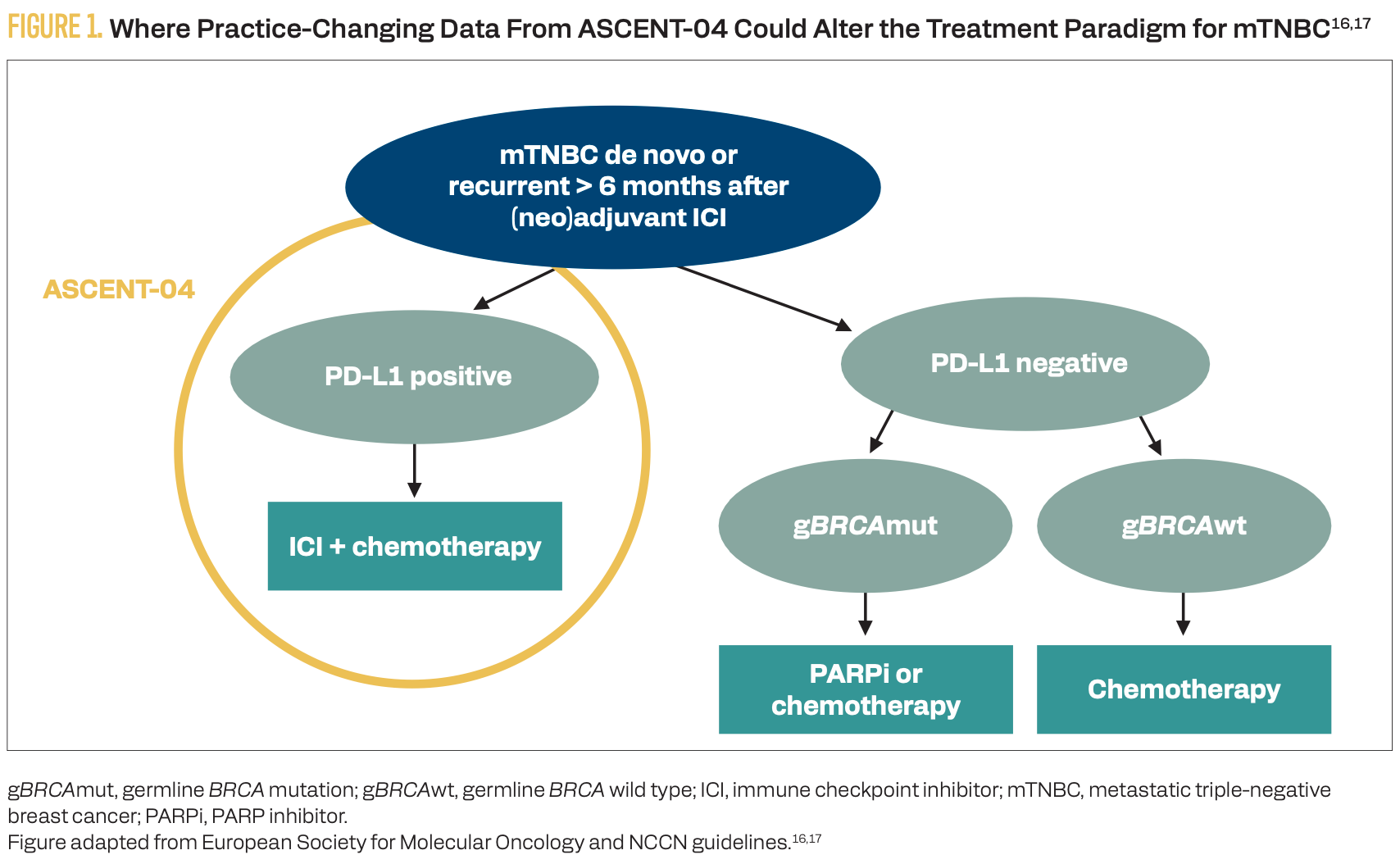
Monotherapy with SG has demonstrated improved efficacy for advanced TNBC in subsequent lines of therapy; it also is being studied in the front line.8,14 The combination of first-line SG with pembrolizumab for patients with advanced unresectable or metastatic, PD-L1–positive (CPS ≥ 10; PD-L1 IHC 22C3 pharmDx assay) TNBC was studied in the phase 3 ASCENT-04/KEYNOTE-D19 study (NCT05382286).15 At a median follow-up of 14 months, SG plus pembrolizumab vs chemotherapy (paclitaxel, nab-paclitaxel, or gemcitabine plus carboplatin) plus pembrolizumab significantly improved PFS (median, 11.2 vs 7.8 months; HR, 0.65; 95% CI, 0.51-0.84; P = .0009), ORR (59.7% vs 53.2%), and DOR (16.5 vs 9.2 months) in the front line.15 The SG plus pembrolizumab group had a similar rate of grade 3 or higher treatment-emergent adverse events (TEAEs; 71% vs 70%) and less than half the rate of TEAEs leading to treatment discontinuation (12% vs 31%) compared with the chemotherapy plus pembrolizumab group.15 These practice-changing data demonstrated superiority of SG plus pembrolizumab over the current standard of care for patients with untreated, PD-L1–positive, advanced TNBC (Figure 1).16,17
FIGURE 1. Where Practice-Changing Data From ASCENT-04 Could Alter the Treatment Paradigm for mTNBC16,17
2/ Frontline datopotamab deruxtecan (Dato-DXd) plus durvalumab has a manageable safety profile and produced a high response rate for patients with advanced TNBC regardless of PD-L1 expression.
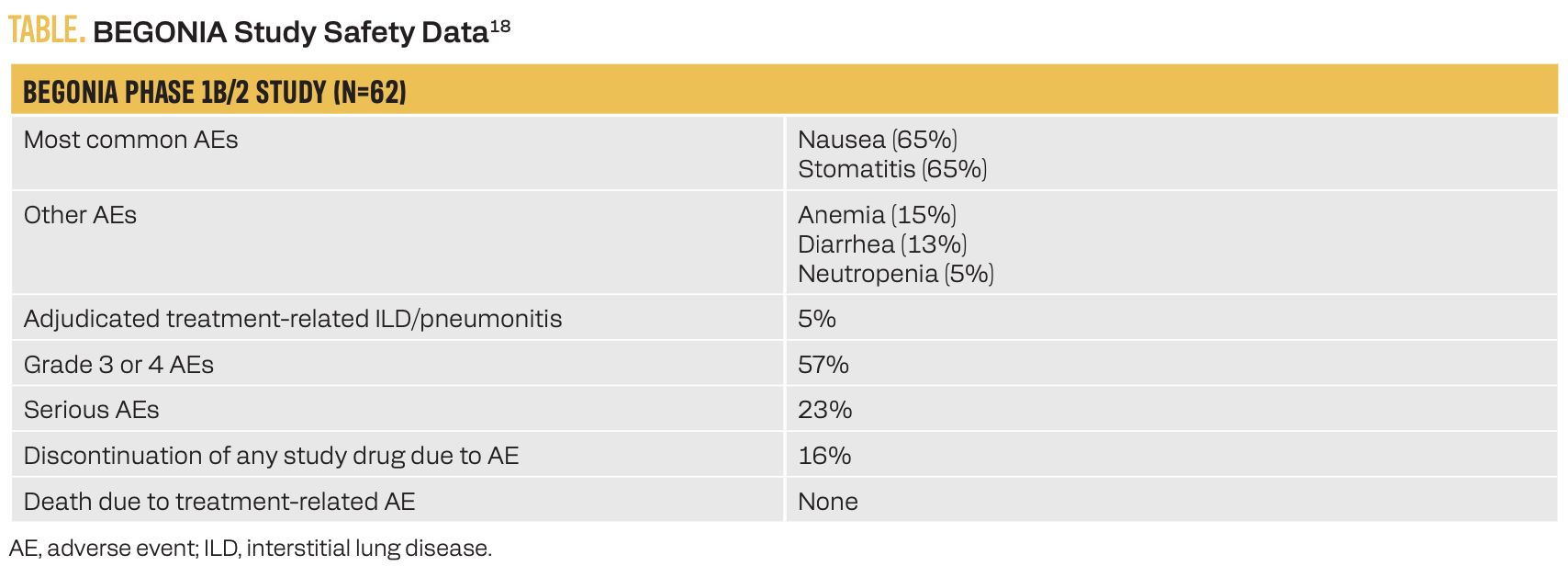
Dato-DXd, another TROP2-targeting ADC, was studied in combination with the PD-L1 inhibitor durvalumab in the first line for patients with advanced TNBC in the phase 1b/2 BEGONIA trial (NCT03742102).18 This ADC/ICI combination did not result in any new safety signals, with 16% of patients discontinuing any study drug due to an adverse event and 5% of patients experiencing treatment- related interstitial lung disease/pneumonitis (Table).18 Secondary efficacy end points for the Dato-DXd plus durvalumab combination for the 62 study patients included a median PFS of13.8 months, an ORR of 79% irrespective of PD-L1 expression level, and a median DOR of 15.5 months. First-line Dato-DXd with or without durvalumab vs chemotherapy of physician’s choice plus pembrolizumab in patients with PD-L1–high, advanced TNBC is being studied in the phase 3 TROPION-Breast05 study (NCT06103864).19
TABLE. BEGONIA Study Safety Data18
3/ Sacituzumab tirumotecan (sac-TMT) monotherapy demonstrated superior efficacy compared with chemotherapy for patients with advanced, previously treated TNBC. It is now being evaluated in the front line as monotherapy and in combination with pembrolizumab.
Sac-TMT (previously SKB264/MK-2870) is an ADC with a TROP2-targeting antibody conjugated to a topoisomerase I inhibitor payload by a novel hydrolyzable linker, allowing for increased drug stability and intracellular (enzymatic) and extracellular (pH-sensitive) cleavage and payload release.20 Sac-TMT monotherapy demonstrated superior efficacy for previously-treated, advanced TNBC compared with chemotherapy in the phase 3 OptiTROP-Breast01 study (NCT05347134).20 In the phase 2 OptiTROP-Breast05 study (NCT05445908) of 41 patients, first-line sac-TMT monotherapy demonstrated an ORR of 70.7% and disease control rate (DCR) of 92.7% for patients with advanced TNBC; the ORR and DCR were similar for patients with PD-L1–low TNBC.21
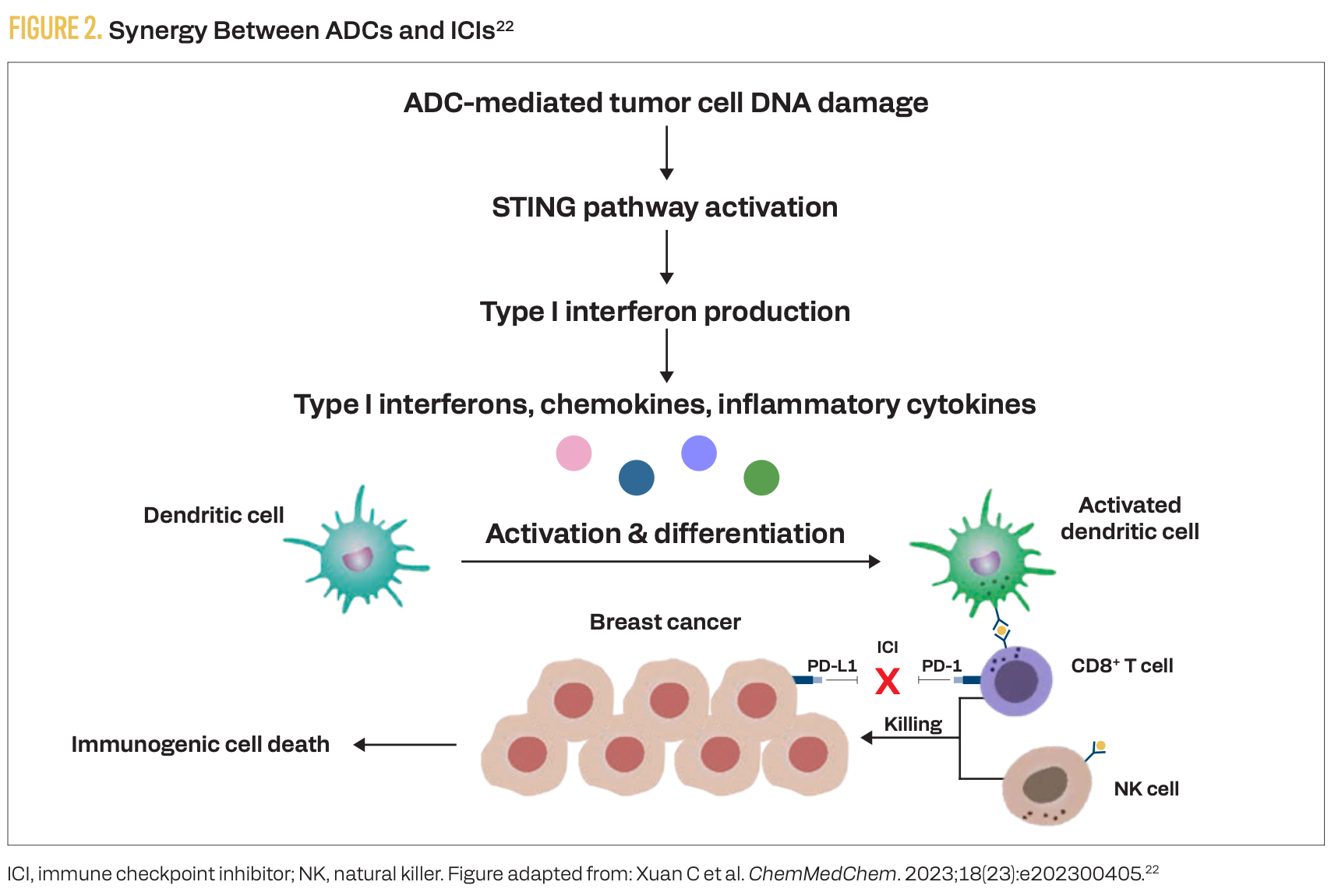
There are multiple mechanisms of synergy between TROP2- targeting ADCs and ICIs (Figure 2), making this combination a rational frontline therapeutic approach for advanced TNBC.22 The phase 3 ASCENT-04 study demonstrated superior efficacy (compared with chemoimmunotherapy) of an ADC plus ICI combination in the front line for PD-L1–positive, advanced TNBC.15 However, PD-L1–negative TNBC remains an area of unmet need.TROP2-targeting ADCs demonstrated efficacy for treatment-naive, advanced TNBC regardless of PD-L1 status in the early-phase BEGONIA and OptiTROP-Breast05 studies.18,21 In the phase 3 TroFuse-011 study (NCT06841354), investigators are evaluating first-line sac-TMT as monotherapy and in combination with pembrolizumab vs treatment of physician’s choice specifically for patients with advanced TNBC that is PD-L1 negative (CPS < 10).23
FIGURE 2. Synergy Between ADCs and ICIs22
Key References:
15. Tolaney SM, Azambuja Ed, Kalinsky K, et al. Sacituzumab govitecan (SG) + pembrolizumab (pembro) vs chemotherapy (chemo) + pembro in previously untreated PD-L1–positive advanced triple-negative breast cancer (TNBC): primary results from the randomized phase 3 ASCENT-04/KEYNOTE-D19 study. J Clin Oncol. 2025;43(suppl 17):LBA109. doi:10.1200/JCO.2025.43.17_suppl.LBA109
18. Schmid P, Wysocki PJ, Ma CX, et al. 379MO Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): updated results from BEGONIA, a phase Ib/II study. Ann Oncol. 2023;34(suppl 2):S337. doi:10.1016/j.annonc.2023.09.556
21. Yin Y, Ouyang Q, Yan M, et al. Sacituzumab tirumotecan (sac-TMT) as first-line treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): initial results from the phase II OptiTROP-Breast05 study. J Clin Oncol. 2025;43(suppl 16):1019. doi:10.1200/JCO.2025.43.16_suppl.1019
For full references list, visit
https://www.gotoper.com/tnbcresource25trop2adcs-postref
To learn more about this topic, including information on how combinations of ADCs plus ICIs are poised to change the landscape of TNBC treatment, go to https://www.gotoper.com/tnbcresource25trop2-activity and https://www.gotoper.com/tnbcresource25adcs-activity
CME Posttest Questions
1/ In the phase 1b/2 BEGONIA trial evaluating immunotherapy and targeted
therapy combinations in advanced TNBC, which of the following was observed in the cohort of patients receiving datopotamab deruxtecan plus durvalumab?
A. Responses were observed mostly in PD-L1–high tumors.
B. Responses were observed mostly in PD-L1–low tumors.
C. Responses were observed in both PD-L1–high and –low tumors.
D. Low response rates were observed regardless of PD-L1 expression.
2/ The ASCENT-04 trial demonstrated a statistically significant and clinically
meaningful benefit using sacituzumab govitecan (SG) plus pembrolizumab for which of the following patient groups with metastatic TNBC?
A. Those with PD-L1–negative tumors only.
B. Those with PD-L1–positive tumors only.
C. Those with both PD-L1–negative and –positive tumors.
D. No benefit was observed with SG.
3/ Based on the primary analysis from the phase 3 ASCENT-04/KEYNOTE-D19 trial, which efficacy outcome(s) showed statistically significant improvement in patients with PD-L1–positive, metastatic TNBC treated with SG plus pembrolizumab compared with those given chemotherapy plus pembrolizumab?
A. Progression-free survival (PFS) only
B. Overall survival (OS) only
C. Both PFS and OS
D. Neither PFS nor OS
Claim Your CME Credit at
https://www.gotoper.com/tnbcresource25trop2adcs-postref
CME Provider Contact Information
Physicians’ Education Resource®, LLC
259 Prospect Plains Road,
Bldg H, Cranbury, NJ 08512
Toll-Free: 888-949-0045
Local: 609-378-3701
Fax: 609-257-0705
info@gotoper.com