Unraveling the Complexities of Proximal-Type Epithelioid Sarcoma of the Vulva
Introduction
Proximal-type epithelioid sarcoma (PES) is one of the most aggressive malignancies, having a high rate of recurrence with poor prognosis. Loss of SMARCB1 is a hallmark that plays a crucial role in pathogenesis and instructing therapeutic approaches. Limited literature on PES underscores the requirement to have a thorough, multidimensional strategy of management.
A 41-year-old woman exhibited a mass without pain in the right labia majora. Modalities of imaging like MRI and PET/CT, along with histopathology and immunohistochemistry, affirmed PES with the loss of SMARCB1. The patient received an extended local excision accompanied by another excision and inguinal lymphadenectomy to achieve local control. Adjuvant radiotherapy was performed but was disrupted at 46 Gy because of severe adverse effects (AEs) such as reactions of the skin and dehiscence of the wound, both of grade 2.
PET/CT scans that were executed after the treatment presented challenges in differentiating recurrence from therapy-associated changes. However, 2 years after the follow-up, the patient was free of disease. This case highlights the significance of a multidimensional strategy incorporating surgery, radiotherapy, and advanced imaging to manage vulvar PES. Challenges like radiation-produced AEs and the molecular pathology role, particularly the loss of SMARCB1, in therapy decision-making, are underscored.
The results emphasize the requirement for long-term strategies of personalized treatment and follow-up in uncommon malignancies such as PES. This case contributes to the limited literature on PES, giving insights into the advancements in diagnosis and treatment. It highlights the importance of resolving the challenges of treatment and offering individualized care for uncommon and aggressive types of cancers.
PES is a rare sarcoma of soft tissues accompanied by a capacity for local recurrence and metastasis that is distant.1,2 It generally produces no pain, with a slowly developing mass, and is usually misunderstood as being a benign tumor; this results in delayed diagnosis, with the potential to impact treatment outcomes.3,4 PES’ scarcity results in noticeable hurdles in diagnosis and therapeutics, necessitating a multidisciplinary approach.5
Due to PES’ rare nature, diagnosis usually relies on various research data, including imaging, biopsy, and immunohistochemistry, to confirm the disease and differentiate it from tumors of soft tissues.4 The aggressive nature can lead to delayed diagnosis, resulting in worse stages of the disease, and intensifying treatment approaches. Surgical intervention is normally accompanied by radiotherapy or chemotherapy, but it is significant to handle PES efficiently.
Epithelioid sarcoma primarily occurs in the extremities. On the other hand, PES principally arises in the pelvic, perineal, and vulvar regions of soft tissues but is greatly uncommon in these areas.6 Vulvar PES is exceedingly rare, with few case reports in the literature.7,8 The deficiency of reported cases constrains conclusive protocols of diagnosis and treatment, which makes clinical management complicated.9
This case report of a 41-year-old woman diagnosed with PES of the vulva describes the diagnosis, treatment, and follow-up of the patients to underscore the clinical characteristics and challenges of managing this rare malignancy. This report emphasizes the early establishment of accurate diagnosis and a multidisciplinary approach for effective outcomes for patients with cases of PES.
Initial Presentation
A 41-year-old woman presented with a painless, mobile, smooth-surfaced mass in the right labia majora with a size of about 5 × 3 cm, and no observable changes in skin. The mass was first noted 9 months before presentation, with its subtle and insidious growth pattern mimicking a benign lesion, delaying diagnosis.
Tumor Location
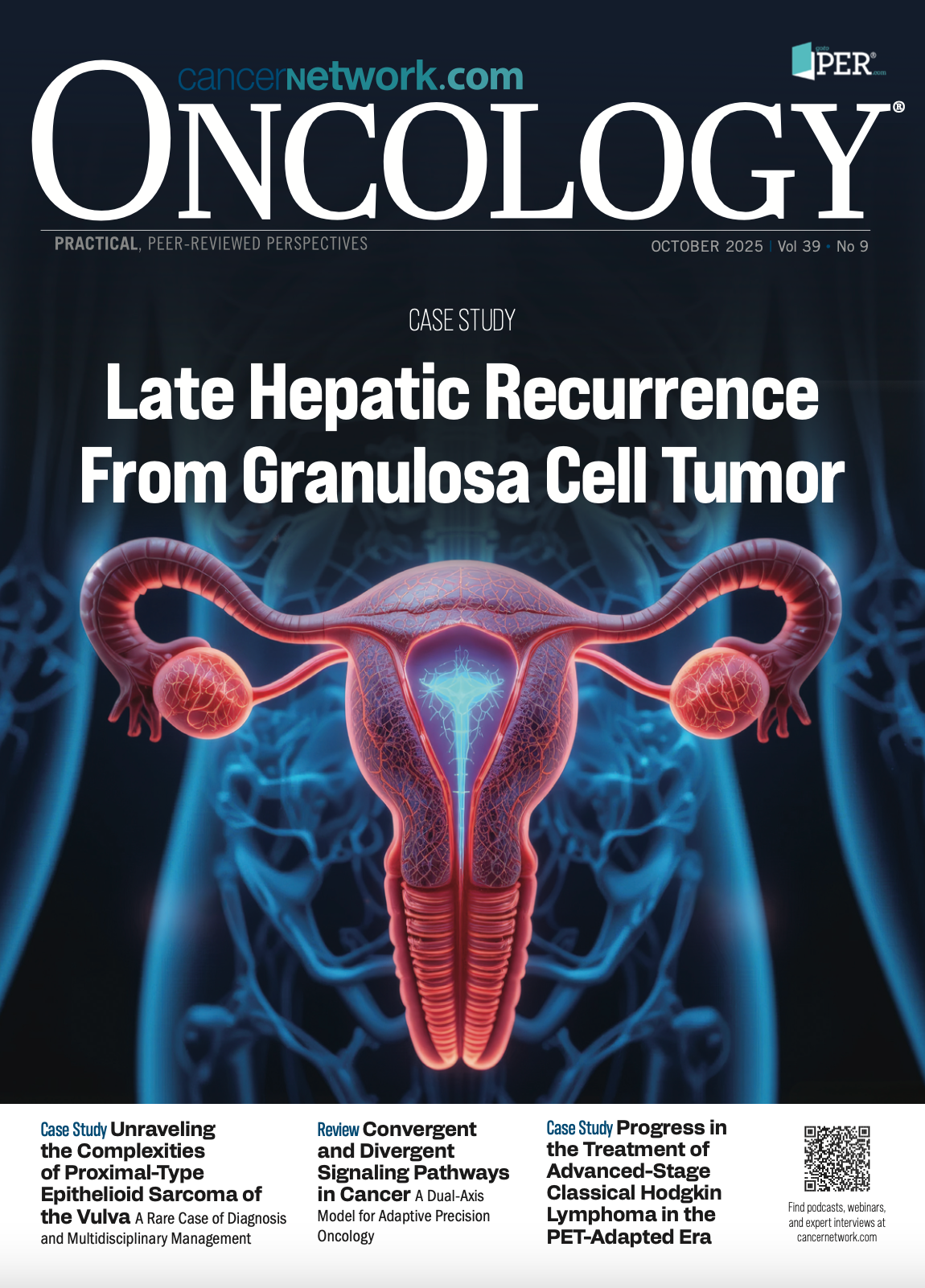
MRI disclosed a 3.7-cm well-circumscribed subcutaneous mass in the right labia majora, having no aggressive hallmarks or related adenopathy, as shown in Figures 1 to 3. After imaging and histopathological confirmation of PES, the patient received treatment that involved surgical excision and adjuvant radiotherapy. The differential diagnosis consisted of superficial angiomyxoma, with less probable chances of angiomyofibroblastoma or myxoid leiomyoma; however, sarcoma was not be conclusively removed.
Figures 1-3.
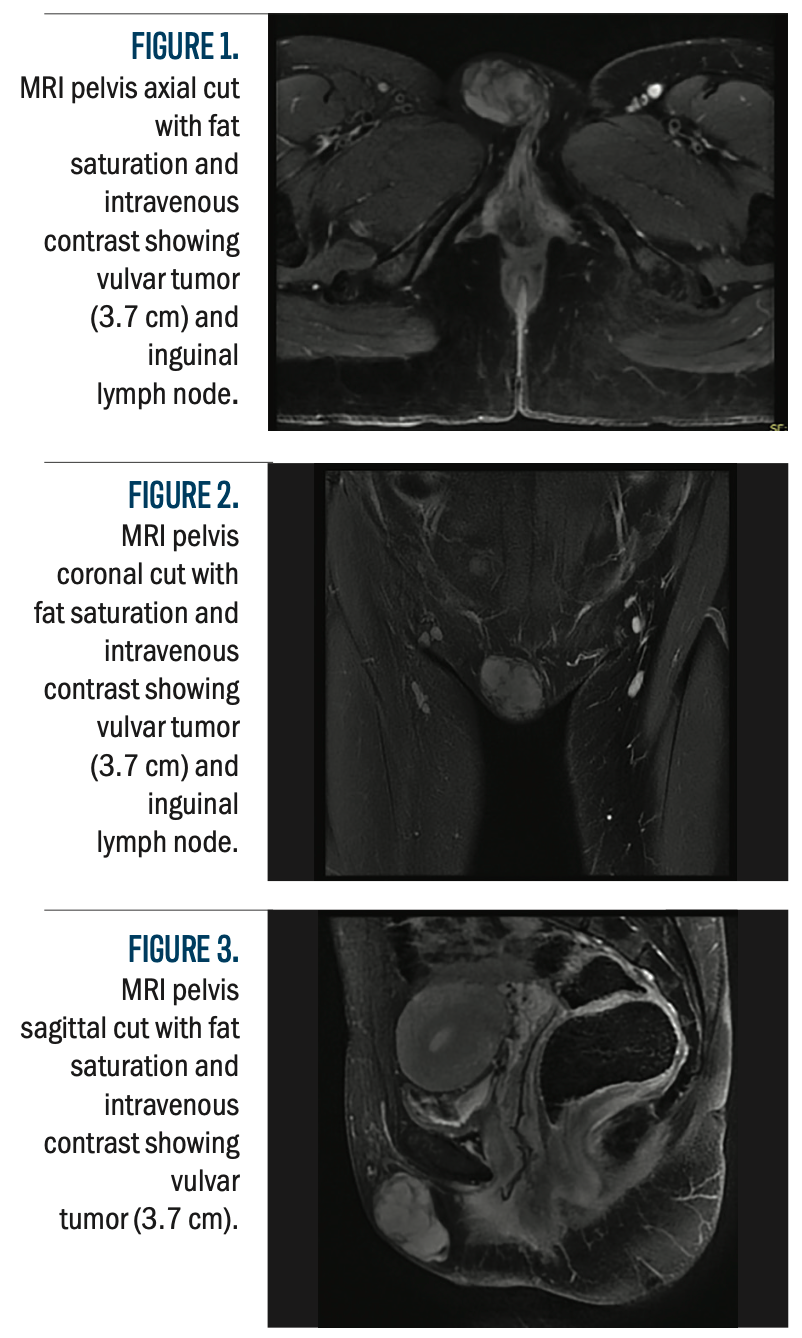
Figure 4a-4c.
Patient Simulation and Immobilization
CT simulation was performed with the patient in a frog-leg supine position to provide reproducibility and comfort at the time of treatment. A Vac-Lok immobilization device was used to decrease movement and enhance the accuracy of placement. Two CT simulations were carried out, one with a full bladder and another with an empty bladder, to compensate for bladder volume change and organ movement. These images were digitally imported into the Eclipse treatment planning system (software version 15.5, Varian Medical Systems, Palo Alto, CA, for treatment planning purposes. To assist in improving accuracy in target definition, the CT images were fused with diagnostic CT and MRI scans to enhance the accuracy in target delineation.
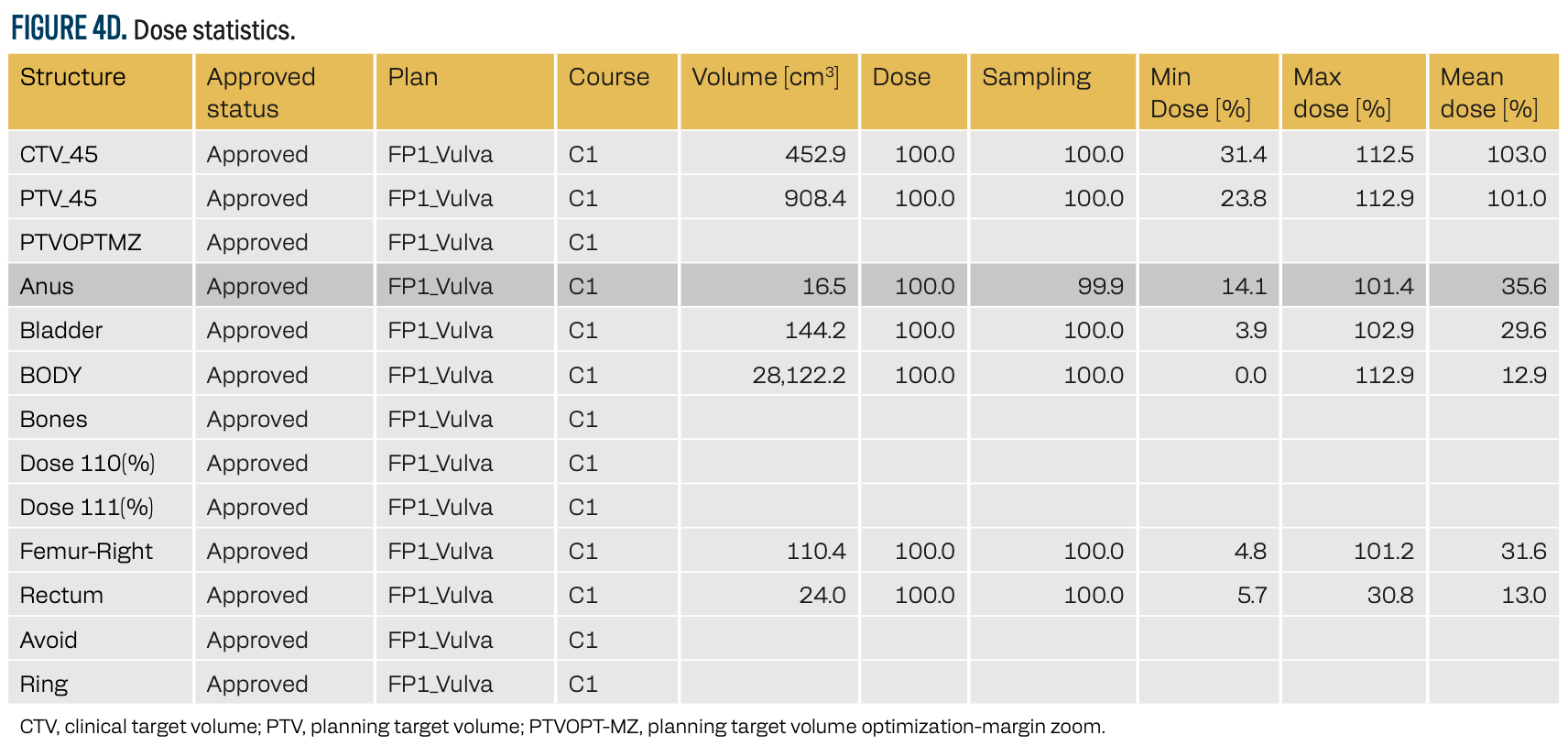
FIGURE 4D. Dose statistics.
Target and Organ-at-Risk Delineation
The clinical target volume encompassed the initial tumor extension, while the planning target volume (PTV) included a 5-mm margin to accommodate setup errors and motion uncertainties. The organs at risk (OARs) considered in treatment planning included the bladder, rectum, femoral heads, and ovaries, ensuring radiation exposure was minimized while maintaining effective tumor control.
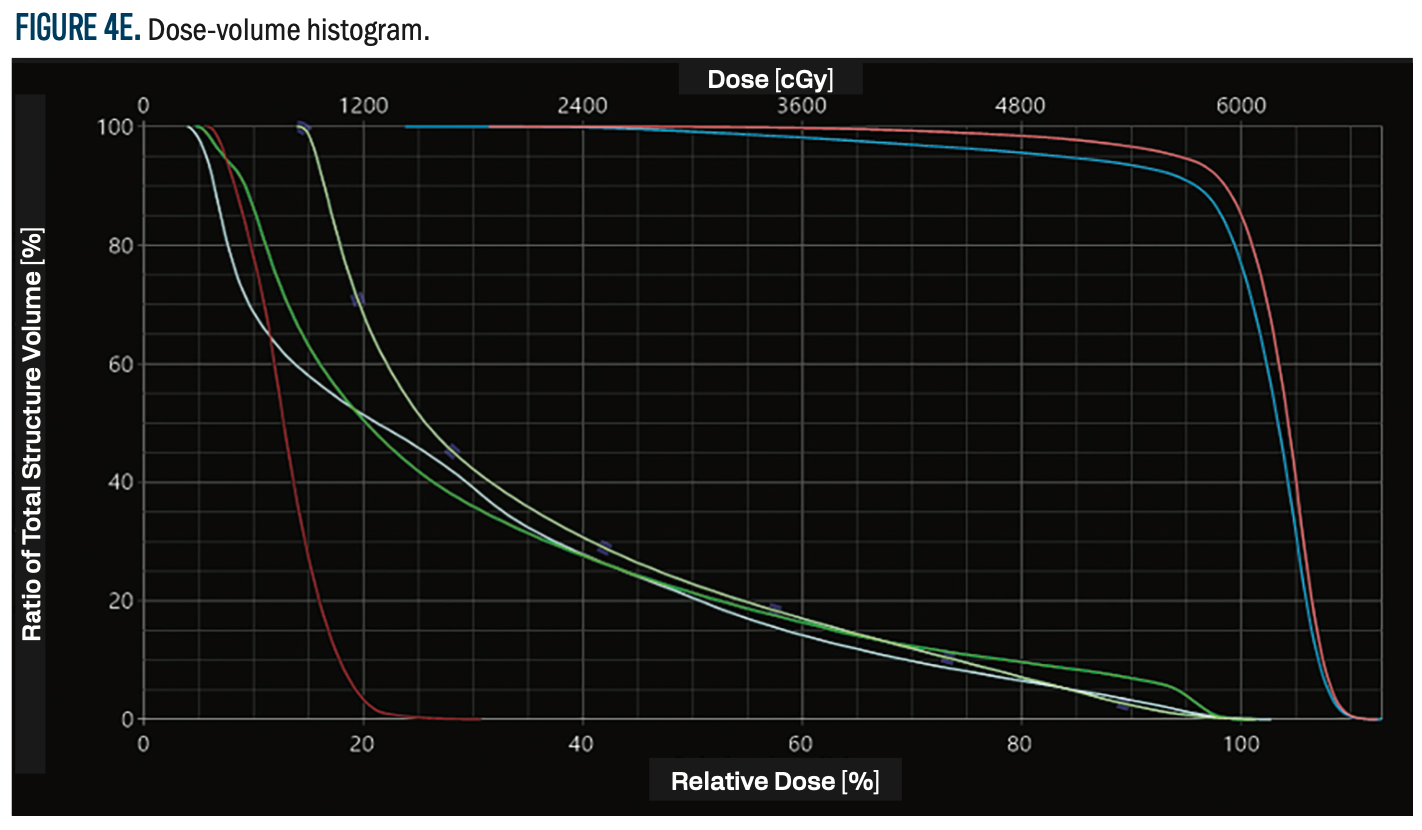
FIGURE 4E. Dose-volume histogram.
Treatment Planning and Dose Prescription
A volumetric-modulated arc therapy (VMAT) plan was developed using the TrueBeam linear accelerator (Varian Medical Systems). The plan was optimized using the Photon Optimizer (version 15.5.11, Varian Medical Systems), with dose calculations performed via the analytical anisotropic algorithm (AAA, version 15.5.11) and a 1-mm dose calculation grid. The prescribed dose was 60 Gy in 30 fractions (2 Gy per fraction) to the tumor bed and right inguinal area, delivered at the 90% isodose line with skin bolus application on alternate days for surface dose coverage. The maximum dose to the PTV was 112%, with an average dose of 101%, ensuring optimal tumor control while adhering to OAR constraints.
Treatment Monitoring and Adjustment
To confirm patient positioning and treatment delivery accuracy, daily cone beam CT (CBCT) scans were conducted during the course of treatment. This image-guided verification enabled ongoing evaluation of treatment accuracy and position. However, due to severe grade 2 skin toxicity and wound dehiscence, treatment was halted at 46 Gy in 23 fractions instead of the planned 60 Gy in 30 fractions.
Dosimetric Assessment
Figure 4 (A-E) shows the dosimetric distribution, with an axial CT simulation cut, illustrating the planned radiation coverage and dose distribution within the target volume and adjacent structures. This figure gives a visual impression of dose conformity, with the assurance that radiation delivery was optimized while protecting critical normal tissues.
Importance of Clinical Suspicion for PES
The age and tumor location in the patient match those in usual cases of PES, predominantly impacting the pelvic area in adults of older age. Though uncommon, vulvar PES is usually seen in older patients rather than the epithelioid sarcoma distal type. Similar to the previously stated cases, the presentation of this patient consisted of a painless mass that might simply be spoiled for the sake of a benign lesion. This case highlights the need for clinicians to maintain a high index of doubt for PES in patients having vulvar masses, specifically those exhibiting late symptoms or lacking of more overt malignancy signs.
Treatment and Follow-Up: Surgical Interventions and Posttherapeutic Challenges
Initial Diagnosis and Surgery
The patient was treated with a wide local excision (WLE) of the vulvar mass on July 27, 2022, the main surgical treatment for PES to obtain complete removal of the tumor with clear margins. Histopathological examination confirmed PES (myxoid variant), and immunohistochemical staining confirmed the diagnosis. The tumor was 5.9 cm at its greatest dimension, and loss of SMARCB1 was identified. The surgical margins were focally involved by malignancy, requiring further treatment.
Lymphadenectomy and Re-excision
Considering the involvement of the surgical margin, the patient was admitted for right superficial inguinal lymphadenectomy and re-excision on September 12, 2022, to evaluate for lymph node involvement and clearance of any remaining malignancy. A histopathological study of 10 removed lymph nodes did not show any malignancy, although dermatopathic changes were found.
A second re-excision of wound margins was done on October 19, 2022, followed by wound closure by a plastic surgery team. The case was then discussed in a gynecologic oncology tumor board on October 25, 2022, with the topic being adjuvant radiotherapy vs surveillance. Due to the aggressive nature of PES, the size of the tumor (5.9 cm), and the focal margin involvement, the patient decided on adjuvant radiotherapy rather than observation.
Treatment Techniques and Approaches
The primary surgical approach included WLE followed by re-excision and lymphadenectomy. The additional re-excision successfully eliminated any residual malignancy, reducing the risk of local recurrence. Lymphadenectomy was performed to evaluate potential nodal metastasis, though no malignant involvement was detected.
Adjuvant Radiotherapy and Complications
The patient underwent VMAT using a TrueBeam linear accelerator, with a prescribed dose of 60 Gy in 30 fractions (2 Gy per fraction) over 6 weeks. The treatment targeted the tumor bed and right inguinal region, ensuring adequate coverage while minimizing exposure to surrounding critical structures. A 5-mm margin was added to the PTV to account for motion and setup errors. To improve dose delivery, a skin bolus was applied on alternate days, enhancing surface dose coverage. Daily image guidance using CBCT was performed to verify patient positioning and ensure accurate radiation delivery.
Despite these measures, the patient developed severe grade 2 skin toxicity and wound dehiscence, which led to treatment discontinuation at 46 Gy in 23 fractions, rather than the planned 60 Gy in 30 fractions. The severity of her reaction was notable, as radiation-induced toxicity in PES remains under
reported due to the rarity of the disease. The patient experienced severe dysuria and a 2-cm wound dehiscence but exhibited no signs of infection or active bleeding. These complications reinforce the importance of individualizing radiation regimens in rare malignancies like PES.
PET/CT Scan and Follow-Up
A PET/CT scan on March 8, 2023, showed fluorodeoxyglucose (FDG) uptake in the inguinal region, raising concerns about possible malignancy. Given the sensitivity of PET/CT in detecting abnormalities, additional clinical correlation was necessary. The patient was further evaluated in the Clinic of Gynecology/Oncology Surgery, where a Papanicolaou test showed no intraepithelial lesions and fine-needle aspiration (FNA) of the suspected inguinal mass confirmed granulomatous inflammation with no malignant cells.
A follow-up PET/CT on May 8, 2024, demonstrated decreasing FDG activity and lesion size, suggesting that prior FDG uptake was likely inflammation or fibrosis rather than tumor recurrence. At 2 years post treatment, the patient remained asymptomatic, with no new masses or pain, and signs of improved skin and mucosal condition.
Discussion
PES of the vulva is an uncommon and aggressive malignancy that has an inappropriate prognosis owing to its high rate of recurrence, with limited literature on its management. The hallmark, loss of SMARCB1, has a key role in pathogenesis and therapeutic approaches. This case stands by the findings stated by Buitron et al and Yue et al, where extended local excision was the principal strategy of treatment, backed by adjuvant radiotherapy.1,6 However, the patient dealt with intense radiotherapy-associated complications, including skin reactions and wound dehiscence of grade 2, resulting in treatment discontinuation, a hurdle not generally reported previously.
On the other hand, cases analyzed by Czarnecka et al obtained successful completion of radiotherapy but underscored recurrence as a notable issue.2 This highlights the demand for treatment planning that is individualized to balance efficiency with the tolerance of the patient. Moreover, the loss of SMARCB1, as seen in this case, is persistently stated as a key feature of PES and is categorized as a possible prognostic and therapeutic marker.1,2
The SMARCB1/INI1 expression loss, being recognized in this case, acts as a conclusive molecular PES hallmark. SMARCB1 can encode a SWI/SNF chromatin subunit remodeling complexes, regulating the expression of genes and sustaining the integrity of chromatin. Its inactivation enhances epigenetic dysregulation, causing tumor phenotypes to be aggressive, with high rates of recurrence, and improper overall prognosis.2,10 This molecular change highlights the requirement for structured tactics of therapies, specifically in aggressive malignancies such as PES. Beyond its diagnostic role, the loss of SMARCB1 is recognized as a convincing target for therapeutic management. Stuy data underscore its link with alleviated EZH2 activity. EZH2 is a histone methyltransferase engaged in a process called oncogenesis. This has resulted in the synthesis of EZH2 inhibitors, like tazemetostat, demonstrating clinical efficiency in cancers lacking SMARCB1.
However, resistance to therapy remains a notable hurdle, which necessitates the discovery of combination regimens that include epigenetic and immune-modulating agents.11
Immunotherapy, which includes immune checkpoint inhibitors, has demonstrated promise by manipulating the immunogenic activity of such tumors, providing a new strategy for improving outcomes.12 This case also explores the recognition of the loss of SMARCB1, reinforcing the aggressive essence of the tumor, underscoring the significance of incorporating molecular profiling into clinical management. Strategies of personalized therapies integrating targeted therapies, immunotherapy, and multimodal treatment strategies could improve prognosis and offer more durable control of the disease. These approaches insist on the demand for ongoing research and clinical trials in uncommon malignancies such as PES.10,12 The case also underscores the crucial role of multidimensional PES management, where furnished imaging and personalized radiotherapy plans are required to improve outcomes.7,13
Sarabia Ochoa et al, Bravo-Taxa et al,and Rodrigues et al showed alignment as well as divergence in outcomes of treatment and strategies of patient management.8,9,14 Similarly, the basic treatment consisted of extended local excision and adjuvant radiotherapy, which reflected the cornerstone of care for vulvar PES. However, the intense radiotherapy-associated AEs in the patient, like grade 2 skin reactions and dehiscence of wounds, were not greatly emphasized in previous cases. Furthermore, the hurdles to interpretation produced by thePET/CT scans after treatment highlight an aspect not discovered before in the literature, especially distinguishing recurrence changes as well as inflammation. Such ideas signify the requirement to have personalized radiotherapy regimens and a completely sophisticated correlation of imaging.14 A long-term robust follow-up is required to recognize any sort of recurrence as well as gaps in metastasis quickly.5
The experience of patients dealing with the immense AEs of radiation signifies the importance of planning personalized treatment. The decision for adjunct radiotherapy should specifically be weighed by factors concerning the condition of health, nature of the tumor, and susceptibility of the patient to secondary radiation AEs.
This case demonstrates that imaging with PET/CT exhibits its utilization to detect recurrence as well as metastasis. However, the findings need to be interpreted with great care, taking into account changes occurring after the treatment and clinical evidence.6,14 The reaction to treatment, shown by the FDG activity regression, provides convincing outlooks for the impactful management of PES. However, this case also sets a demand on the existing barriers in the treatment of PES and the significant requirement for further research in continuing the development of impactful treatment strategies and personalized therapies for this rare, aggressive disease.6
The present case report was similar in the clinical presentation, the histopathological results, and the treatment methods employed in earlier PES reports. The patient presented with a painless vulvar mass, which is consistent with and similar to the results of other published cases with a myxoid variant for PES histopathology. Therefore, the present case was managed with a WLE coupled with adjuvant radiotherapy, which is recommended in the recent guidelines set for practice.1,7,15,16
The patient’s experience with intense reactions to radiation and the rare uptake pattern of FDG on PET/CT scans require more inquiry and discussion. The unusual uptake pattern of FDG on PET/CT, which suggests malignancy despite the negative FNA, highlights the hurdles in the interpretation of imaging results in the case of posttreatment inflammation and the demand for more research into the utilization of PET/CT in the monitoring of PES.6,17 This case report also highlights the importance of long-lasting follow-up and the necessity for new studies on other impactful treatments for PES.
In conclusion, the present case underscores the diagnostic and therapeutic complications of the management of PES of the vulva, which is an uncommon and aggressive malignancy. Primary imaging disclosed a properly circumscribed subcutaneous mass of 3.7 cm in the right labia majora, mostly sustained with superficial angiomyxoma, having differential diagnoses consisting of angiomyofibroblastoma and myxoid leiomyoma. However, sarcoma could not be eliminated. Impactful management indicates multidimensional strategy, incorporating expertise from gynecologists, oncologists, radiation oncologists, and pathologists. Given the high recurrence rate of PES, meticulous long-lived follow-up and studies into pathogenesis, targeted therapies, and enhanced treatment protocols to advance patient outcomes and life quality.
Future Directions
Future studies in PES should concentrate on resolving the molecular and therapeutic hurdles presented by the loss of SMARCB1. The development of targeted therapies, like EZH2 inhibitors, presents a convincing aspect to tackle epigenetic dysregulation linked with this rarity. Moreover, incorporating advanced modalities of imaging, such as PET tracers particular to tumor biology, might enhance diagnostic precision and distinguish recurrence from changes after the treatment. Disclosing substitute modalities of treatment, including proton therapy, may minimize the intense AEs seen with traditional radiotherapy. Cooperative multicenter research is critical to producing strong data for assessing such strategies and recognizing biomarkers predicting the response to treatment and enhancing outcomes in this uncommon and
aggressive malignancy.
Recommendations
- Use PET/CT regulalry for follow-up to detect recurrence.
- Incorporate targeted therapies such as EZH2 inhibitors
and discover combination regimens. - Cooperate on multicenter research for standardizing
protocols of treatment. - Enhance treatments for balancing effectiveness and
minimizing AEs.
Corresponding Author
Fatimah M. Kaabi, MBBS
e-mail: Kaabi.fatima73@gmail.com
ORCID: 0000-0001-6476-3419
References
1. Buitron EZ, Panduro DAV, Luna DM. Clinicopathological characteristics, treatment, and survival in patients diagnosed with proximal-type epithelioid sarcoma: a case report and systematic review. Cureus. 2022;14(12):e32962. doi:10.7759/cureus.32962
2. Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers (Basel). 2020;12(8):2112. doi:10.3390/cancers12082112
3. Echchaoui A, Sadrati Y, Elbir Y, et al. Proximal-type epithelioid sarcoma: a new case report and literature review. Pan Afr Med J. 2016;24;238. doi:10.11604/pamj.2016.24.238.8535
4. DeLaney TF, Harmon DC, Sikora K, Hornicek FJ. Soft tissue sarcomas. In: Price P, Sikora K, eds. Treatment of Cancer. CRC Press; 2020:434-458.
5. Shkolnik E, Cai D. Characterization of a rare case of vulvar epithelioid sarcoma with local recurrence and metastases. Am J Clin Pathol. 2021;156(suppl 1):S81. doi:10.1093/ajcp/aqab191.171-S81
6. Yue Y, Lu Y, Ma X, Tang Z, Cheng Y. Ultrasonography findings of proximal‑type epithelioid sarcoma of the vulva: a case report. Mol Clin Oncol. 2018;9(5):507-510. doi:10.3892/mco.2018.1708
7. Chung HW, Shin SJ. The treatment of pazopanib on vulvar epithelioid sarcoma: a case report and review of literature. 대한산부인과학회학술발표논문집. 2014;100:373-373.
8. Sarabia Ochoa R, García de la Torre JP, Amezcua Recover A. Proximal epithelioid sarcoma of the vulva: a rare neoplasm: a clinicopathological case. Iberoam J Med. 2024;6(1):17-22. doi:10.53986/ibjm.2024.0002
9. Bravo-Taxa M, Taxa-Rojas L, López-Blanco A. Proximal epithelioid sarcoma of the vulva: a case report and review of the literature. Clin Surg Res Commun. 2021;5(3):43-50. doi:10.31491/CSRC.2021.09.081
10. Cooper GW, Hong AL. SMARCB1-deficient cancers: novel molecular insights and therapeutic vulnerabilities. Cancers (Basel). 2022;14(15):3645. doi:10.3390/cancers14153645
11. Ngo C, Postel-Vinay S. Immunotherapy for SMARCB1-deficient sarcomas: current evidence and future developments. Biomedicines. 2022;10(3):650. doi:10.3390/biomedicines10030650
12. Kang HG, Koh J, Kim TM, et al. Molecular and treatment characteristics of SMARCB1 or SMARCA4-deficient undifferentiated tumor: retrospective case series. Cancer Res Treat. 2024;56(3):967-971. doi:10.4143/crt.2023.1308
13. Dy PMR, Yturralde EMH, Luna JTP. Proximal-type epithelioid sarcoma of the vulva: a case report. Acta Med Philipp. 2025;59(5):82-87.
doi:10.47895/amp.vi0.10057
14. Rodrigues AI, Lopes HI, Lima O, Marta S. Proximal-type epithelioid sarcoma—unusual presentation: unilateral vulvar mass. BMJ Case Rep. 2015:2015:bcr2014208488. doi:10.1136/bcr-2014-208488
15. Tan YL, Ong W, Tan JH, Kumar N, Hallinan JTPD. Epithelioid sarcoma of the spine: a review of literature and case report. J Clin Med. 2023;12(17):5632. doi:10.3390/jcm12175632
16. Watanabe S, Iwata Y, Saito K, Sugiura K. Proximal-type epithelioid sarcoma arising in the abdomen of a young woman: a case report. J Dermatol. 2022;49(1):e26-e27. doi:10.1111/1346-8138.16196
17. Kumara RMMSK, Jeevani U, Prabodini MAM, Sulochana W, Badra HP. Metastatic proximal-type epithelioid sarcoma presenting as a chest lesion with a pathological fracture of the humeral neck. Int J Case Rep Images. 2022;13(2):137-141. doi:10.5348/101345Z01RK2022CR
18. Yahiro S, Fujimoto T, Fujita I, et al. Proximal-type epithelioid sarcoma in pubic region expressing L-type amino acid transporter 1: a case report. SAGE Open Med Case Rep. 2022;10:2050313X211067917. doi:10.1177/2050313X211067917
19. Xia S, Wu W, Ma L. Proximal-type epithelioid sarcoma of the perineum: a case report and literature review. Front Oncol. 2023;13:1057466. doi:10.3389/fonc.2023.1057466