3 Things You Should Know About Single-Agent ADCs in TNBC Treatment
Explore the promising role of antibody-drug conjugates targeting TROP2 in treating metastatic triple-negative breast cancer and improving patient outcomes.
The experts.

LEARNING OBJECTIVES
Upon successful completion of this activity, you should be better prepared to:
- Describe the mechanistic rationale for utilizing antibody-drug conjugates (ADCs) to target TROP2 in the treatment of patients with metastatic triple-negative breast cancer (TNBC)
- Evaluate recent evidence supporting the evolving role of ADCs in the treatment of patients with metastatic TNBC
- Develop evidence-based strategies for integrating ADCs in the treatment of patients with metastatic TNBC
RELEASE DATE: October 1, 2025
EXPIRATION DATE: October 1, 2026
Accreditation/Credit Designation
Physicians’ Education Resource®, LLC, is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physicians’ Education Resource®, LLC, designates this enduring material for a maximum of 0.25 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Acknowledgement of commercial support
This activity is supported by an educational grant from Gilead Sciences, Inc.
Off-Label Disclosure and Disclaimer
This activity may or may not discuss investigational, unapproved, or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a health care professional relative to diagnostic, treatment, or management options for a specific patient’s medical condition. The opinions expressed in the content are solely those of the individual faculty members, and do not reflect those of PER® or any company that provided commercial support for this activity.
Instructions for Participation and How to Receive Credit
1. Read this activity in its entirety.
2. Go to https://www.gotoper.com/tnbc25resource-postref to access and complete the posttest.
3. Answer the evaluation questions.
4. Request credit using the drop-down menu.
YOU MAY IMMEDIATELY DOWNLOAD YOUR CERTIFICATE.
In triple-negative breast cancer (TNBC), a lack of amplification or overexpression of HER2and no expression of either the estrogen or progesterone receptors limit the potential targets to treat this disease. Antibody-drug conjugates (ADCs) that target TROP2 are helping to address this unmet need. Here are 3 things you should know about single-agent ADCs used to treat TNBC.
1/ TROP2 is an actionable target in TNBC.
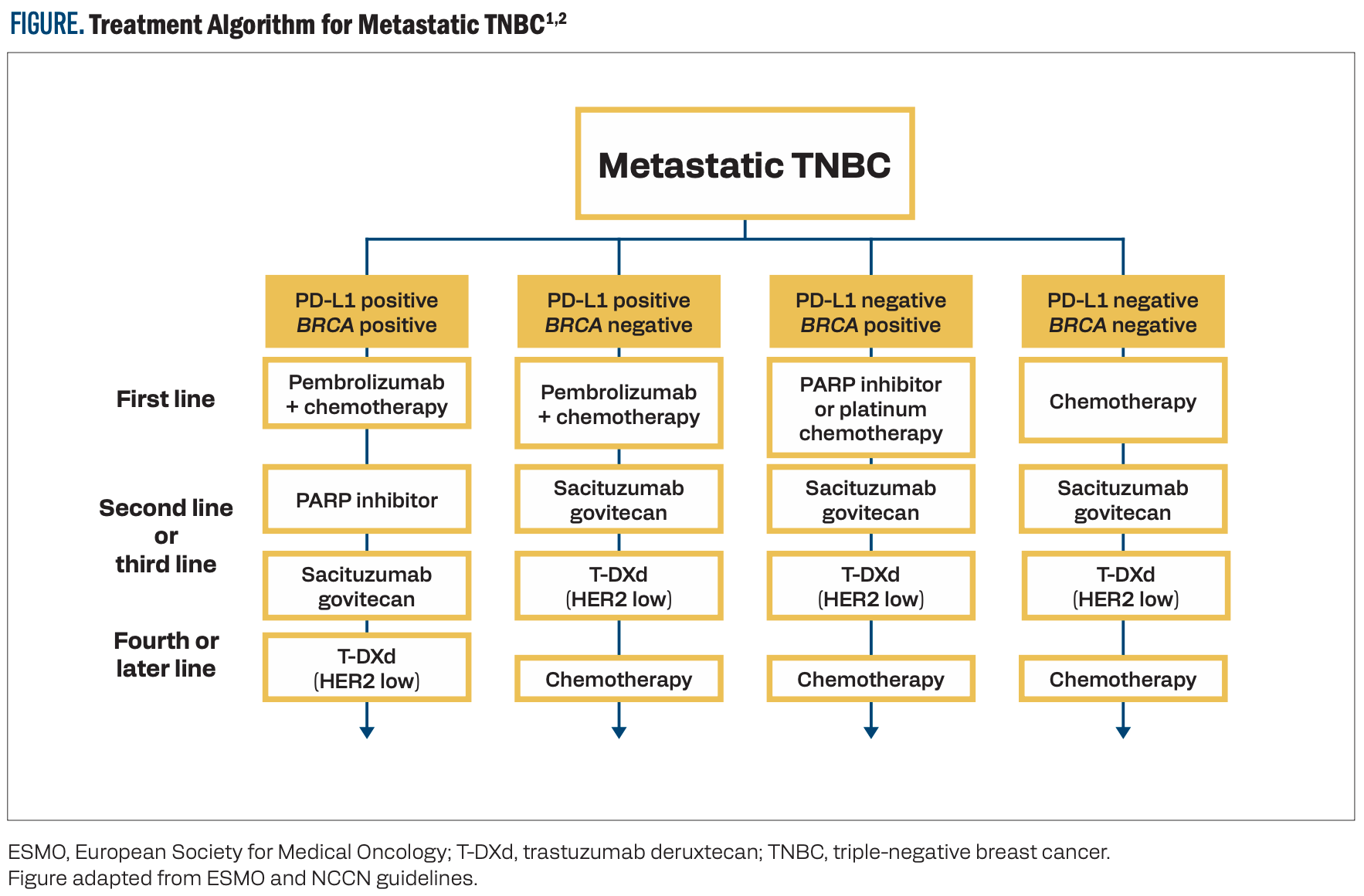
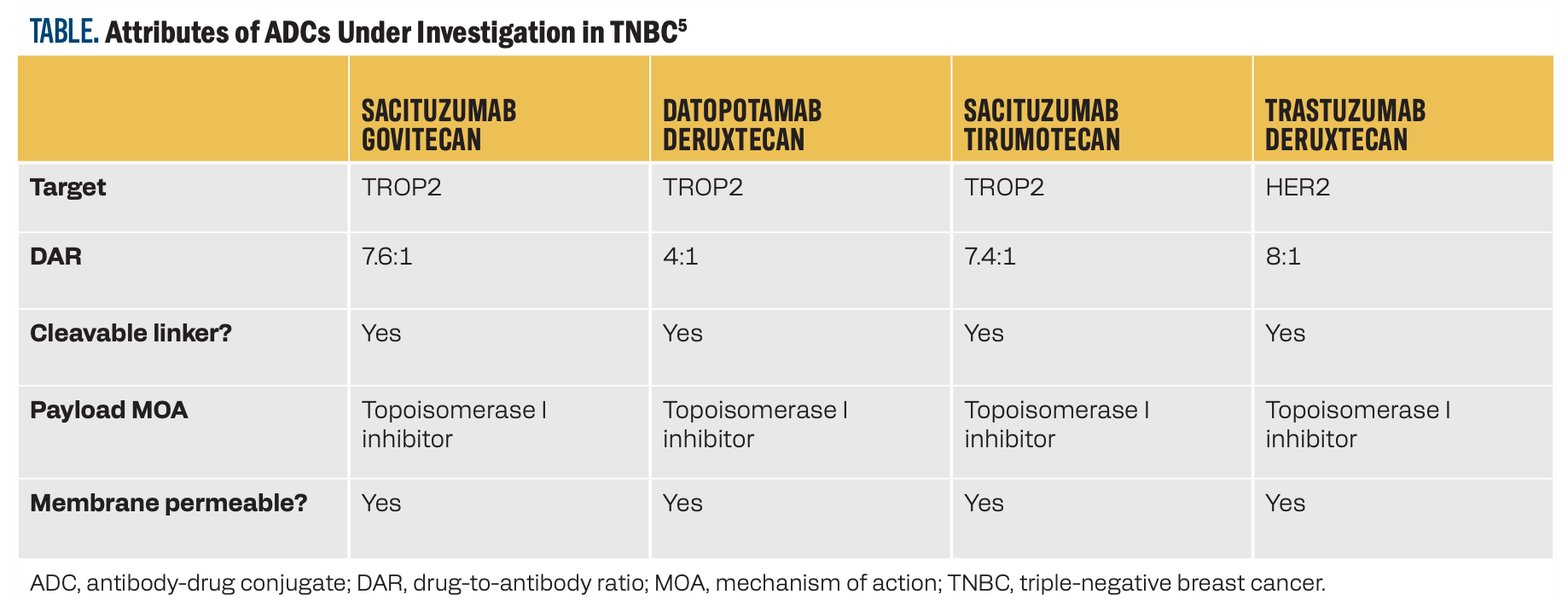
Prognosis is poor for patients with TNBC, and the need for additional treatment options is high. Current algorithms for treating patients with metastatic TNBC hinge on the expression levels of PD-L1 and the presence of a BRCA mutation (Figure).1,2 Once frontline chemotherapy combinations are exhausted, additional targeted therapeutic options are necessary. TROP2 demonstrates low expression in normal tissues but is overexpressed in 93% of TNBC tumors, making it an effective target for novel therapeutics.3 Sacituzumab govitecan (SG) is a TROP2-targeting ADC approved for use in TNBC.4 Datopotamab deruxtecan (Dato-DXd) and sacituzumab tirumotecan (sac-TMT) are additional ADCs that bind to TROP2 and are under investigation in this disease state.
FIGURE. Treatment Algorithm for Metastatic TNBC1,2
2/ TROP2- and HER2-targeting ADCs are under investigation as monotherapy in TNBC.
Several ADCs have been evaluated in late-phase clinical trials as monotherapy in previously treated and systemic treatment–naive patients with TNBC (Table).5 SG is an established second- or third-line therapy based on the results of the phase 3 ASCENT trial (NCT02574455), which demonstrated a progression-free survival (PFS) benefit with SG monotherapy vs physician’s choice of chemotherapy in patients with relapsed or refractory metastatic TNBC (4.8 vs 1.7 months; HR, 0.41; 95% CI, 0.33-0.52).6 Overall survival (OS) was also increased in the SG arm vs the chemotherapy arm (11.8 vs 6.9 months; HR, 0.51; 95% CI, 0.42-0.63). The most common adverse events (AEs) of grade 3 or higher in the SG and chemotherapy arms, respectively, were neutropenia (51% vs 33%), leukopenia (10% vs 5%), diarrhea (10% vs < 1%), anemia (8% vs 5%), and febrile neutropenia (6% vs 2%).7 Frontline use of SG monotherapy is under investigation vs standard-of-care chemotherapy in the phase 3 ASCENT-03 trial (NCT05382299) in patients with PD-L1–positive metastatic TNBC.8 According to a recent news release, the ASCENT-03 trial has met its primary PFS end point; results are anticipated at an upcoming meeting.9
TABLE. Attributes of ADCs Under Investigation in TNBC5
Single-agent treatment with Dato-DXd demonstrated efficacy as a second- or later-line therapy in 44 patients with TNBC in the phase 1 TROPION-PanTumor01 trial (NCT03401385).10 Among these patients, the objective response rate (ORR) was 31.8% (95% CI, 18.6%-47.6%). The median duration of response was 16.8 months, and the median PFS was 4.4 months (95% CI, 3.0-7.3). The most common treatment-emergent AE was stomatitis (72.7%). Grade 3 events occurred in 11.4% of patients with TNBC. Frontline Dato-DXd monotherapy is currently under evaluation vs chemotherapy in patients with TNBC in the ongoing phase 3 TROPION-Breast02 trial (NCT05374512).11
In the phase 3 OptiTROP-Breast01 trial (NCT05347134), in previously treated patients with TNBC, sac-TMT monotherapy demonstrated a median PFS of 6.7 months vs 2.5 months with chemotherapy (HR, 0.32; 95% CI, 0.24-0.44; P < .00001). The median OS with sac-TMT was not reached vs 9.4 months with chemotherapy (HR, 0.53; 95% CI, 0.36-0.78; P = .0005).12 In the sac-TMT arm, the ORR was 45.4% vs 12.0% with chemotherapy. The most common AEs of grade 3 or 4 in the sac-TMT and chemotherapy arms, respectively, were decreased neutrophil count (34.6% vs 47.0%), decreased white blood cell count (27.7% vs 36.4%), decreased platelet count (13.1% vs 3.8%), anemia (29.2% vs 6.1%), and stomatitis (10.0% vs 0.8%).
Approximately one-third of TNBC tumors can be classified as HER2 low (immunohistochemistry [IHC] staining score of 1+ or 2+ with negative reflex in situ hybridization testing), or HER2ultralow (IHC 0 with faint staining in up to 10% of cells), adding the option of treatment with the HER2-targeting ADC trastuzumab deruxtecan (T-DXd).13 In the phase 3 DESTINY-Breast04 trial (NCT03734029), 557 patients with HER2-low metastatic breast cancer who were previously treated with chemotherapy,including 63 with hormone receptor–negative disease, were randomly assigned 2:1 to receive T-DXd monotherapy or the physician’s choice of chemotherapy.14 T-DXd provided a significant improvement in median PFS vs chemotherapy in the overall population (HR, 0.50; 95% CI, 0.40-0.63; P < .001) and in both the hormone receptor–positive (HR, 0.51; 95% CI, 0.40-0.64) and hormone receptor–negative cohorts (HR, 0.46; 95% CI, 0.24-0.89). Interstitial lung disease (ILD) occurred in 45 patients receiving T-DXd, mostly grade 1 or 2. Three patients experienced grade 5 ILD.
3/ As more ADCs become available for patients with TNBC, optimal sequencing must be determined.
SG and T-DXd have overlapping FDA approvals for clinical indications. The efficacy of SG was demonstrated in the ASCENT trial, which enrolled 468 patients with metastatic TNBC.7 Efficacy of T-DXd for HER2-low TNBC was demonstrated in a cohort of 63 patients in the DESTINY-Breast04 trial, which enrolled patients with both hormone receptor–positive and hormone receptor–negative disease.14
To address the question of which ADC should be used first, a real-world study was conducted on 4030 tumor specimens from patients with HER2-negative breast cancer who had received treatment with T-DXd, SG, or both.15 There was no significant difference in time on treatment (TOT) between T-DXd and SG for the 1368 patients who had hormone receptor–negative /HER2-negative disease and received only one ADC (4.1 vs 3.4 months, respectively; HR, 0.912; 95% CI, 0.783-1.063; P = .237). Although cross-resistance may occur due to the similar chemotherapy payload of these ADCs, both are often given sequentially. In the cohort of 780 patients in this study who received both ADCs, no benefit to TOT or OS was seen with T-DXd first vs SG first, with one exception: Patients who had hormone receptor–negative and HER2-null disease (IHC 0 with no visible staining) experienced a significant benefit when treated with SG first vs T-DXd first, both in regards to TOT (11.7 vs 7.4 months; HR, 0.478; 95% CI, 0.333-0.685; P < .0001) and OS (19.7 vs 11.8 months; HR, 0.478; 95% CI, 0.303-0.756; P = .001).
Key References
5. Li N, Yang L, Zhao Z, et al. Antibody-drug conjugates in breast cancer: current evidence and future directions.Exp Hematol Oncol. 2025;14(1):41. doi:10.1186/s40164-025-00632-9
11. Dent RA, Cescon DW, Bachelot T, et al. TROPION-Breast02: datopotamab deruxtecan for locally recurrent inoperable or metastatic triple-negative breast cancer. Future Oncol. 2023;19(35):2349-2359. doi:10.2217/fon-2023-0228
12. Yin Y, Fan Y, Ouyang Q, et al. Sacituzumab tirumotecan in previously treated metastatic triple-negative breast cancer: a randomized phase 3 trial. Nat Med. 2025;31(6):1969-1975. doi:10.1038/s41591-025-03630-w
For full reference list, visit
https://www.gotoper.com/tnbc25resource-postref
CME Posttest Questions
1/ The cytotoxic payloads of sacituzumab govitecan, datopotamab deruxtecan, and sacituzumab tirumotecan all use what mechanism of action?
A. DNA alkylation
B. Microtubule inhibition
C. Thymidine synthase inhibition
D. Topoisomerase I inhibition
2/ Based on the OptiTROP-Breast01 trial, which efficacy outcome(s) showed statistically significant improvement with sacituzumab tirumotecan vs chemotherapy in pretreated metastatic triple-negative breast cancer (mTNBC)?
A. Progression-free survival (PFS) only
B. Overall survival (OS) only
C. Both PFS and OS
D. Neither PFS nor OS
3/ A 51-year-old woman presents to your clinic with de novo mTNBC. Her breast cancer is PD-L1 positive with no germline BRCA1/2 mutation. She receives pembrolizumab plus nab-paclitaxel but has disease progression after 10 months. Immunohistochemistry of a biopsy specimen of a new metastatic lesion reveals HER2 1+ expression; the combined positive score is 12. Based on the strength of available evidence, what would be the next best step in the treatment plan for this patient?
A. Datopotamab deruxtecan
B. Gemcitabine/carboplatin
C. Sacituzumab govitecan
D. Sacituzumab + pembrolizumab
E. Trastuzumab deruxtecan
Claim Your CME Credit at https://www.gotoper.com/tnbc25resource-postref
To learn more about this topic, including information on real-world data of sacituzumab govitecan in pretreated mTNBC, go to https://www.gotoper.com/tnbc25resource-trop2-activity and https://www.gotoper.com/tnbc25resource-adcs-activity
CME Provider Contact Information
Physicians’ Education Resource®, LLC
259 Prospect Plains Road,
Bldg H, Cranbury, NJ 08512
Toll-Free: 888-949-0045
Local: 609-378-3701
Fax: 609-257-0705
info@gotoper.com