AI-Powered Scout Platform Could Enhance Oncology Decision-Making With Data- and Expert-Driven Insights
Artificial intelligence (AI) has slammed into the elements of everyday life in some fashion, and the oncology profession has not been excluded. Scout, an AI-powered assistant developed to streamline access to expert clinical insight and research-based commentary, launched in May 2025 and is accessible on the desktop and mobile versions of OncLive.com, CancerNetwork.com, and TargetedOncology.com.
But what is Scout truly capable of? After real-world clinical scenarios were posted into Scout, oncology leaders reviewed the AI platform’s responses, weighed their validity and quality, and touched on how Scout can best complement their clinical practice.
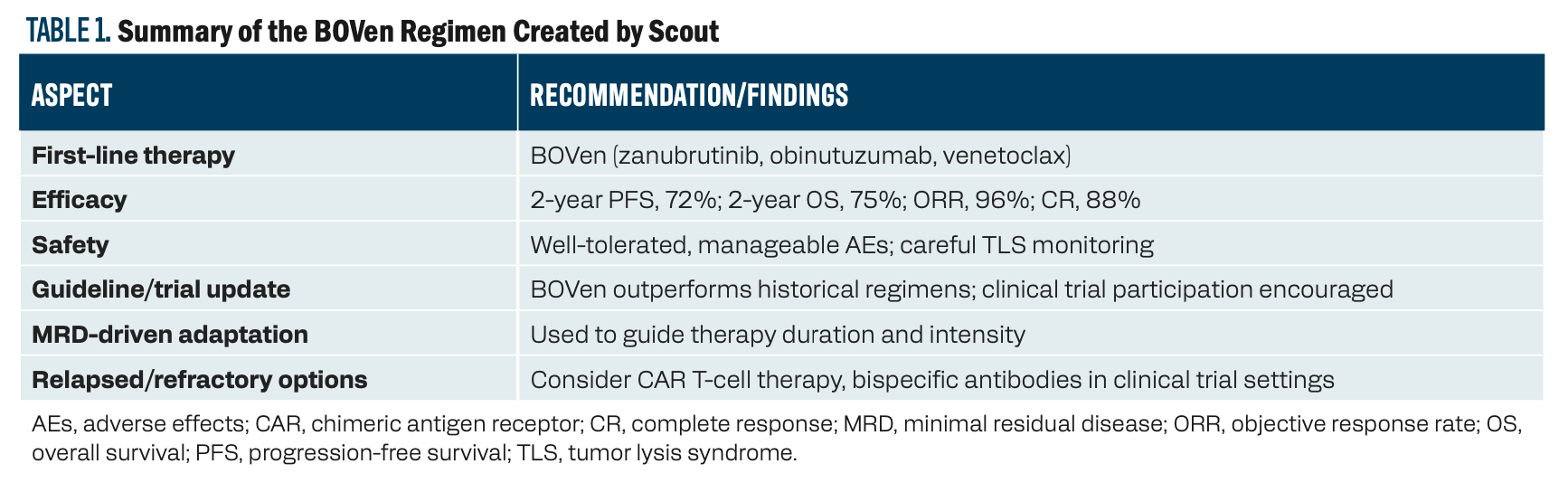
Key aspects and recommendations include the following. See Table 1 for more information.

1 Impact of TP53 mutation: TP53-mutant MCL is considered high-risk and is associated with poor survival and low response rates to conventional chemoimmunotherapy, including intensive regimens and autologous stem cell transplant.
2. Best current treatment option: The most promising regimen is the BOVen combination: zanubrutinib (Brukinsa; a [Bruton tyrosine kinase] inhibitor), obinutuzumab (Gazyva; an anti-CD20 antibody), and venetoclax (Venclexta; a BCL-2 inhibitor).
a. In a recent phase 2 trial, this regimen achieved a 2-year progression-free survival (PFS) rate of 72% and a 2-year overall survival rate of 75% in [patients with] previously untreated, TP53-mutant MCL. The overall response rate was 96%, with an 88% complete metabolic response rate.
b. The regimen is outpatient-based, well-tolerated, and uses a minimal residual disease (MRD)–driven approach to adapt therapy duration and intensity.
3. Safety and tolerability: The BOVen regimen was generally well-tolerated. The most common adverse effects (AEs) were diarrhea, neutropenia, and infusion reactions, with manageable rates of serious AEs. Tumor lysis syndrome risk was mitigated by careful venetoclax ramp-up.
4. Clinical trial and guideline updates: The BOVen regimen is the first to show such promising results specifically in TP53-mutant MCL, outperforming historical controls [eg, 2-year PFS rate of 20% with intensive chemoimmunotherapy and 55% with ibrutinib (Imbruvica)-based regimens].
a. There is a growing consensus among experts and guidelines to prioritize novel agent–based, chemotherapy-free regimens for TP53-mutant MCL, and to consider clinical trial enrollment whenever possible.
5. Additional considerations: MRD assessment is increasingly used to guide treatment adaptation and de-escalation, aiming to minimize toxicity while maintaining efficacy.
a. For relapsed/refractory cases or those not responding to novel agents, CAR [chimeric antigen receptor] T-cell therapy and bispecific antibodies are under investigation and may be considered in select patients.
TABLE 1. Summary of the BOVen Regimen Created by Scout
In summary, for a symptomatic 65-year-old [man] with stage IVA TP53-mutant MCL, the BOVen regimen is currently the most evidence-based and promising frontline option, with strong support from recent clinical trials and expert recommendations, as highlighted by Anita Kumar, [MD, of Memorial Sloan Kettering Cancer Center] and other leading investigators.
“I tried it out with a couple of clinical cases, and it came up with what I would consider the correct answer,” Vose said in response to the above prompt and reply. “It did seem to take a bit longer to generate the answers than ChatGPT, for example. It says references 1 to 3, but it did not actually give me the references. That would be my only bad comment.”
Clinical Scenario
Robert Jones is a 68-year-old [man] with an 8-year history of IgG-κmultiple myeloma, initially diagnosed in 2017. His initial workup revealed International Staging System (ISS) stage III, Revised-ISS stage III disease with high-risk cytogenetics (t(4;14), del(17p)). He was initially treated with bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone (VRd), followed by an autologous stem cell transplant [ASCT]. He achieved a very good partial response and was maintained on lenalidomide, but relapsed 2 years later.
He has since received multiple lines of therapy, including daratumumab (Darzalex)/pomalidomide (Pomalyst)/dexamethasone (DPd), isatuximab-irfc (Sarclisa)/carfilzomib (Kyprolis)/dexamethasone (IsaKd), and, most recently, a clinical trial with a B-cell maturation antigen (BCMA)–targeted bispecific antibody. Despite these treatments, his disease has progressed, with his M [monoclonal]-spike rising from 0.8 g/dL to 2.5 g/dL over the past 3 months.
His recent labs show [the following]:
- Hemoglobin [level]: 9.2 g/dL (down from 11.5 g/dL)
- Creatinine [level]: 1.8 mg/dL (up from 1.2 mg/dL)
- Corrected calcium [level]: 10.8 mg/dL
- Serum β2-microglobulin [level]: 6.2 mg/L
- M-spike: 2.5 g/dL
- Free light chain ratio: 105 (κ: 450 mg/L; λ: 4.3 mg/L)
- Bone marrow biopsy: 40% clonal plasma cells
- PET/CT scan: Multiple new lytic bone lesions in the spine and pelvis, with a focal lesion in the left humerus
Jones’ performance status is ECOG 2 due to increasing fatigue and bone pain. He has an extensive history of neuropathy from previous bortezomib and requires careful management of his renal function. What is the next best course of treatment for this patient?
Scout Response
For a 68-year-old [man] with high-risk, relapsed/refractory IgG-κ multiple myeloma (t(4;14), del(17p)), who has progressed after VRd, ASCT, lenalidomide maintenance, DPd, IsaKd, and a BCMA-targeted bispecific antibody, and now presents with worsening cytopenias, renal dysfunction, new bone lesions, ECOG 2, significant neuropathy, and renal impairment, the next best evidence-based treatment options should consider both disease biology and comorbidities:
1 GPRC5D-targeted bispecific antibody (eg, talquetamab [Talvey]): After failure of BCMA-targeted therapy, switching to a GPRC5D-targeted bispecific antibody such as talquetamab is supported by current expert consensus. Ajay Nooka, [MD, of Emory Winship Cancer Institute] and colleagues highlight that talquetamab is particularly suitable for patients with poor organ function, cytopenias, and high infection risk, as it is associated with a lower rate of severe infections compared with BCMA bispecifics (grade 3 infections less than 10% vs greater than 50% for BCMA bispecifics) and does not require Pneumocystis jirovecii pneumonia prophylaxis as monotherapy. This makes it a strong option for patients with significant comorbidities, including renal dysfunction and cytopenias, as in your case.
2. CELMoDs (eg, iberdomide, mezigdomide): For heavily pretreated, high-risk patients, CELMoDs (cereblon E3 ligase modulators) are emerging as potential options, especially if not previously used. Compassionate use or clinical trial enrollment may be considered, as discussed by Ajai Chari, [MD, of the University of California, San Francisco] and colleagues. These agents can be combined with other drugs the patient has previously tolerated, but careful consideration of cytopenias and renal function is needed.
3. CAR T-cell therapy (ciltacabtagene autoleucel [Carvykti] or idecabtagene vicleucel [Abecma]): CAR T-cell therapy remains an option for fit patients, even in later lines. However, Surbhi Sidana, [MD, of Stanford Medicine] and panelists emphasize that frailty, organ dysfunction, and performance status are critical in decision-making. For patients with ECOG 2, significant neuropathy, and renal impairment, the risks of CAR T (including severe infections, neurotoxicity, and nonrelapse mortality) must be weighed against potential benefits. Bridging therapy and frailty assessment are essential, and some centers may still consider CAR T for high-risk, functional patients if comorbidities are manageable.
Key considerations for your patient include the following. See Table 2 for the full results.
Table 2. Summary of Treatment Options Created by Scout

GPRC5D bispecifics (talquetamab) are preferred due to lower infection risk and better tolerability in the context of cytopenias and renal dysfunction.
CELMoDs may be considered if available and if the patient’s cytopenias are manageable.
CAR T-cell therapy is an option if the patient’s performance status and comorbidities allow but may not be ideal given current frailty and organ dysfunction.
In summary, the most evidence-based and practical next step is to pursue a GPRC5D-targeted bispecific antibody, such as talquetamab, with supportive care for cytopenias and renal dysfunction, and consider CELMoDs or CAR T-cell therapy only if the patient’s performance status and comorbidities improve or are manageable.

“CELMoDs are not FDA approved and won’t be until 2026/2027,” Richter said, emphasizing the current investigational use of this class of agents. “Talquetamab is my recommendation for treatment, but there are other options, including selinexor [Xpovio]-based therapy.”
How Supportive Care Methods Can Improve Oncology Outcomes
Experts discussed supportive care and why it should be integrated into standard oncology care.