Recap: Experts Discuss NRG1 Fusions as Key Oncogenic Drivers for Certain Patients With Lung Cancer
D. Ross Camidge, MD, PhD, and Alexander I. Spira, MD, PhD, discussed the use of NRG1 fusions as being a driver for patients with lung cancer.
Data regarding clinical, pathologic, and molecular features of NRG1 fusion–positive lung cancers are relatively limited given the rarity of NRG1 aberrations and their recent discovery in this tumor type.1-3
With NRG1 fusions first discovered in lung cancers in 2014,4 limited data exist on tumors harboring these oncogenes. Therefore, effectiveness of systemic therapies to treat patients with NRG1 fusion–positive lung cancers is also limited when compared with other lung cancer subtypes.
In a recent Between the Lines presentation hosted by CancerNetwork®, D. Ross Camidge, MD, PhD, and Alexander I. Spira, MD, PhD, discussed details from the eNRGy1 Global Multicenter Registry, which sought to contribute pertinent data to a central registry on patients with NRG1 fusion–positive lung cancers.
“Here we are in the 21st century and we are describing new diseases,” Camidge, director of the Thoracic Oncology Clinical and Clinical Research Programs at the University of Colorado in Aurora, explained. “[Research] is showing that NRG1 fusion–positive small cell lung cancer is out there, it’s real, it’s super rare, but it’s not Bigfoot.”
“We need a lot more information,” Spira, director of Virginia Cancer Specialists Research Institute and the Phase I Trial Program in Alexandria, Virgina, emphasized. “The [sample sizes in trials] are really small, and as some of these newer studies develop, I think we’ll certainly learn a lot more.”
Spira and Camidge spoke about this lung cancer subtype in the context of findings from the eNRGy1 registry, which collected data from over 22 centers across 9 countries to evaluate clinical, pathologic, molecular, and response data for patients with NRG1 fusion–positive lung cancers.
Data on NRG1 Fusion–Positive Tumors From eNRGy1
Patient eligibility for the eNRGy1 registry required a pathologically confirmed NRG1 fusion–positive lung cancer diagnosis. The investigators set out to collect data across different disease features including clinicopathologic characteristics and tumor immunophenotype.
Data for 110 patients were included in the research. Patients had a median age of 64 years (range, 29-88) and 59% of patients were female. Most patients were either Asian (52%) or White (46%), and 57% were never smokers. Most patients (71%) had nonmetastatic disease. For the 44 patients with metastatic disease diagnosed at any time, the most common sites of metastases were the lung (71%), bone (34%), or lymph nodes (23%).
When evaluated by disease stage, median overall survival (OS) was not reached (95% CI, 51.5-undefined) for patients with stage I disease (n=26), 52.9 months (95% CI, 38.8-undefined) with stage II disease (n=19), 78.2 months (95% CI, 11.0-undefined) with stage III disease (n=13), and 15.5 months (95% CI, 10.3-64.5) for patients with stage IV disease (n=24).
NRG1 fusions were mostly detected using RNA-based testing (74%), with DNA-based tests used in only about one-quarter of the registrants (26%). Specifically, the most frequently used RNA-based assay was an anchored multiplex polymerase chain reaction (PCR) test (62%), followed by reverse transcription-PCR (27%). Next-generation sequencing (NGS; 52%) was the most commonly used DNA-based assay.
Camidge is a proponent of RNA-based testing and said a combination approach could help identify more patients with these extremely rare fusions. “Using an RNA- in addition to a DNA-based extraction to do your next-generation sequencing really boosts the sensitivity for picking up these rare fusions,” he explained.
Of note, tumor PD-L1 status was known for only 42% of the 110 patients included in the study, but Spira said this was probably due to a data set that’s a bit older. “It’s almost unheard of now that you would have PD-L1 unknown,” he said.
High PD-L1 expression, defined as 50% or greater, was observed in only 4% of patients with known PD-L1 status (2 of 46). Most tumors had either no PD-L1 expression (72%) or PD-L1 expression of 1% to 49% (24%).1
Median tumor mutational burden (TMB) in NRG1 fusion–positive lung cancers was 0.9 mutations/megabase (range, 0-2.6), which is low when compared with different fusion statuses (TABLE).
Camidge continued, “If I have a never smoker with adenocarcinoma who has PD-L1 [expression] of 100%, that’s a red flag that they have a driver oncogene. You have to resist the temptation to rush [to treating with] single-agent immunotherapy and wait for the molecular [testing] to come back, if possible.”
When activity of systemic therapies was evaluated among eligible patients with metastatic disease, response rates were low. Targeted therapy with afatinib induced responses in 25% of the treatment patients (5 of 20) and represented the greatest efficacy, followed by single-agent immunotherapy (20%; 1 of 5), taxane-based chemotherapy (14%; 1 of 7), platinum doublet–based chemotherapy (13%; 2 of 15), and chemoimmunotherapy (0%; 0 of 9) (FIGURE).
FIGURE. Activity of Systemiv Therapy by Regimen
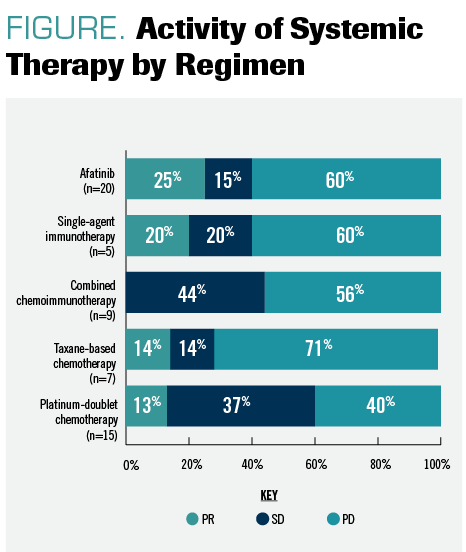
“The implication here is that these patients, at least in this data set, are not really responding to many of the standard therapies,” Camidge explained.
Future Implications for NRG1 Fusion–Positive Lung Cancer
“It’s the beginning,” Camidge emphasized, regarding the emergence of NRG1 and the need to further develop testing capabilities for this specific fusion. For greater precision approaches, clinicians should examine testing panels used at their centers to ensure they are checking their patient’s tumors for these fusions ahead of agents becoming available for treatment.
Future research is needed to further explore the impact of NRG1 fusions and to identify effective systemic therapeutic options for patients.
Although the positivity rate for NRG1 fusion–positive lung cancers is only 0.2%, as Camidge explained, that rate is significant for the patients whose tumors harbor these oncogenes. Spira continued, “The only challenge of this journey is this is rare. We say ALK is rare, but that’s 4%. Here, we’re [talking] less than 1%.”
“Even if it’s only that small percentage, we don’t need to remind everybody the difference it makes for those individual patients,” Spira explained. “Even if it’s a fraction of a percent, for that 1 patient, it’s a game-changer, and I think that’s the important thing.”
References
1. Drilon A, Duruisseaux M, Han JY, et al. Clinicopathologic features and response to therapy of NRG1 fusion-driven lung cancers: The eNRGy1 Global Multicenter Registry. J Clin Oncol. 2021;39(25):2791-2802. doi:10.1200/JCO.20.03307
2. Drilon A, Somwar R, Mangatt BP, et al. Response to ERBB3-directed targeted therapy in NRG1-rearranged cancers. Cancer Discov. 2018;8(6):686-695. doi:10.1158/2159-8290.CD-17-1004
3. Jonna S, Feldman RA, Swensen J, et al. Detection of NRG1 gene fusions in solid tumors. Clin Cancer Res. 2019;25(16):4966-4972. doi:10.1158/1078-0432.CCR-19-0160
4. Fernandez-Cuesta L, Plenker D, Osada H, et al. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov. 2014;4(4):415-422. doi:10.1158/2159-8290.CD-13-0633
EP: 1.NRG1 Fusion–Driven Lung Cancers: Clinicopathologic Features and Response to Therapy
EP: 2.Key Takeaways From the eNRGy1 Global Multicenter Registry Study
EP: 3.Considering the Role of NRG1 Fusions in Lung Cancer Management
EP: 4.NRG1 Fusion-Driven Lung Cancer: Future Directions in Care
EP: 5.Preclinical Data Show Promise of Seribantumab in HER3-Positive Cell Lines
EP: 6.Recap: Experts Discuss NRG1 Fusions as Key Oncogenic Drivers for Certain Patients With Lung Cancer
