Recap: Optimizing Therapy in Frontline Multiple Myeloma Not Eligible for Transplant
A panel of experts discuss strategies for optimizing first-line therapy for transplant-ineligible patients with newly diagnosed multiple myeloma.

Older patients who present with transplant-ineligible multiple myeloma are often characterized by a wide range of ages, fitness levels, and disease progression, making this a challenging disease subtype for clinicians to manage. At an Around the Practice presentation hosted by CancerNetwork®, a panel of experts discussed how to optimize first-line therapy for this patient group. The discussion was moderated by C. Ola Landgren, MD, PhD, professor of medicine, chief of the Myeloma Program, and Experimental Therapeutics Program lead at the Sylvester Comprehensive Cancer Center of the University of Miami in Miami, Florida.
Challenges in Treating Transplant-Ineligible Disease
The 5-year overall survival (OS) rate for patients with multiple myeloma that has spread to distant parts of the body is 54%.1 One single-institution study presented at the 2019 American Society of Clinical Oncology Annual Meeting reported that OS in multiple myeloma can range from more than 9 years for patients with stage I disease to 3.5 years for those with stage III.2 For the roughly two-thirds of patients with myeloma who are older than 65 years, those rates can be even lower.3 And although autologous stem cell transplants have contributed to rising survival rates in this disease, fewer than 20% of older patients receive a stem cell transplant.4
Yet another challenge is the lack of rigorous trials that include older patient subsets. Assuming patients could access the locations, usually at academic and tertiary referral centers, they still might not be eligible for the trials, as 65 years is a common age limit used in eligibility criteria, although some trials may allow patients aged up to 75 years. Therefore, many myeloma trials are not representative of older, frail patients, so extrapolating data for the very elderly must be done with caution.
Assessing Fitness
Older patients who are medically fit may not be good transplant candidates because of the treatment’s effect on the body. “If you have a 70-year-old [patient] in good condition, you [may] give high-dose chemotherapy and they age dramatically over the span of a few weeks,” Cristina Gasparetto, MD, professor of medicine at Duke Cancer Institute in Durham, North Carolina, explained. “Very fit patients can lose everything during the transplant because they don’t have any reserve.”
Peter Voorhees, MD, director of outreach for hematologic malignancies in the Plasma Cell Disorder Program, Department of Hematologic Oncology, at the Levine Cancer Institute at Atrium Health in Charlotte, North Carolina, therefore said determining a patient’s level of fitness is essential. “Age is not what we should be using to determine transplant eligibility. We have to do a good job of determining that level of fitness; having that conversation with the patient in the clinic room and giving them an ECOG performance status of 1 is not sufficient,” he elaborated.
Voorhees pointed out that the International Myeloma Working Group (IMWG) has its own frailty score, “which is useful and is associated with worse outcomes and higher rates of severe toxicity with therapy for those who are frail. I think we need to start employing [that] in the clinic more often.” The IMWG classifies patients as fit, intermediate fit, and frail using a simplified geriatric assessment tool that takes into account patient age, comorbidities, and activities of daily living (ADL), including instrumental ADL.
Access to Care
Gasparetto said it’s common for academic teaching hospitals to not see many patients in the older patient group, as this group is typically seen in community settings. As such, she pointed out that several elements should be considered to ensure that patients can continue through the duration of treatment; these would include, for instance, a social support network, ability to follow through on routine lab checks, and whether they can travel for their treatment. “Sometimes we [as clinicians] forget about that,” she added.
Compared with those seen in academic settings, older patients with myeloma treated in the community may not have the same access to advanced treatments, such as transplant therapy, clinical trials, and novel systemic therapies.
Patient Communication
Craig C. Hofmeister, MD, MPH, associate professor in the Department of Hematology and Medical Oncology at Emory University in Atlanta, Georgia, communicating with older patients can present its own challenges. In fact, different communications strategies are often needed depending on if the patient is aged 40, 60, or 85 years. “My patients who are 85 years old often change quickly in terms of tolerance and adverse effects,” Hofmeister said. “All of a sudden, it’s not that easy to get hold of them, bring them in, see them.”
Comorbidities
In addition to the increasing frailty among older patients, more comorbidities are also to be expected and, in turn, increases in adverse events associated with therapy. Comorbidities may include a history of myocardial infarction, congestive heart failure, peripheral vascular disease, and dementia, among others.
Response Assessment in Transplant-Ineligible Myeloma
When assessing patients for response to systemic therapy in this setting, the panel discussed different strategies, which may include regular blood work and repeated bone marrow biopsies and imaging.
Hofmeister commented that for standard-risk patients, tracking the serum monoclonal protein light chains is usually easiest when testing. “For your oligosecretory and nonsecretory [disease], assessment depends more on imaging. For truly nonsecretory disease, bone marrow biopsies [are necessary] if they have nothing else to track and no myeloma bone disease,” said Hofmeister. “That’s my standard in most patients.” He added that 90% to 95% of the time, he’s just doing blood tests and doesn’t repeat imaging or marrow tests.
Voorhees says repeat bone marrow testing for transplant-ineligible patients is not likely unless they have oligosecretory and nonsecretory disease. If they are in a clinical trial, or there is some indication for testing, repeat testing may be necessary, “particularly if they have high-risk disease features cytogenetically.”
Regarding repeated imaging, Voorhees said, “for the patient with extramedullary disease who has extensive uptake on initial staging PET/CT, particularly if they have high-risk disease features cytogenetically, I would typically like to repeat PET/CT imaging after the
initial therapy prior to transitioning to a maintenance platform, to determine that all sites of disease have responded.”
Gasparetto agreed with Voorhees’s approach. “I think that the patients with PET-positive disease are in a different category and going from PET-positive to PET-negative is extremely important. For transplant-ineligible patients, if I don’t make any changes in therapy, I don’t do the bone marrow but rather monitor the blood markers.”
On the other hand, Landgren does more biopsies. “I like to repeat biopsies if I intend to give more effective therapy [based on] a prognostic indicator. The data tell me that cytogenetics is less powerful than achieving a deep response.”
Selecting Acceptable Treatment Regimens
Next steps in treatment greatly depend on the regimen, according to Voorhees. He
detailed patient outcomes from the SWOG S0777 trial (NCT00644228), in which
patients who were not treatment planned for transplant received 24 weeks of a 3-drug regimen—bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone—followed by lenalidomide/dexamethasone (Rd) vs Rd alone until disease progression.5 Results showed that the addition of bortezomib led to greater progression-free survival and OS.
“I wouldn’t use the dexamethasone until disease progression, but they did deescalate therapy in that trial,” said Voorhees.
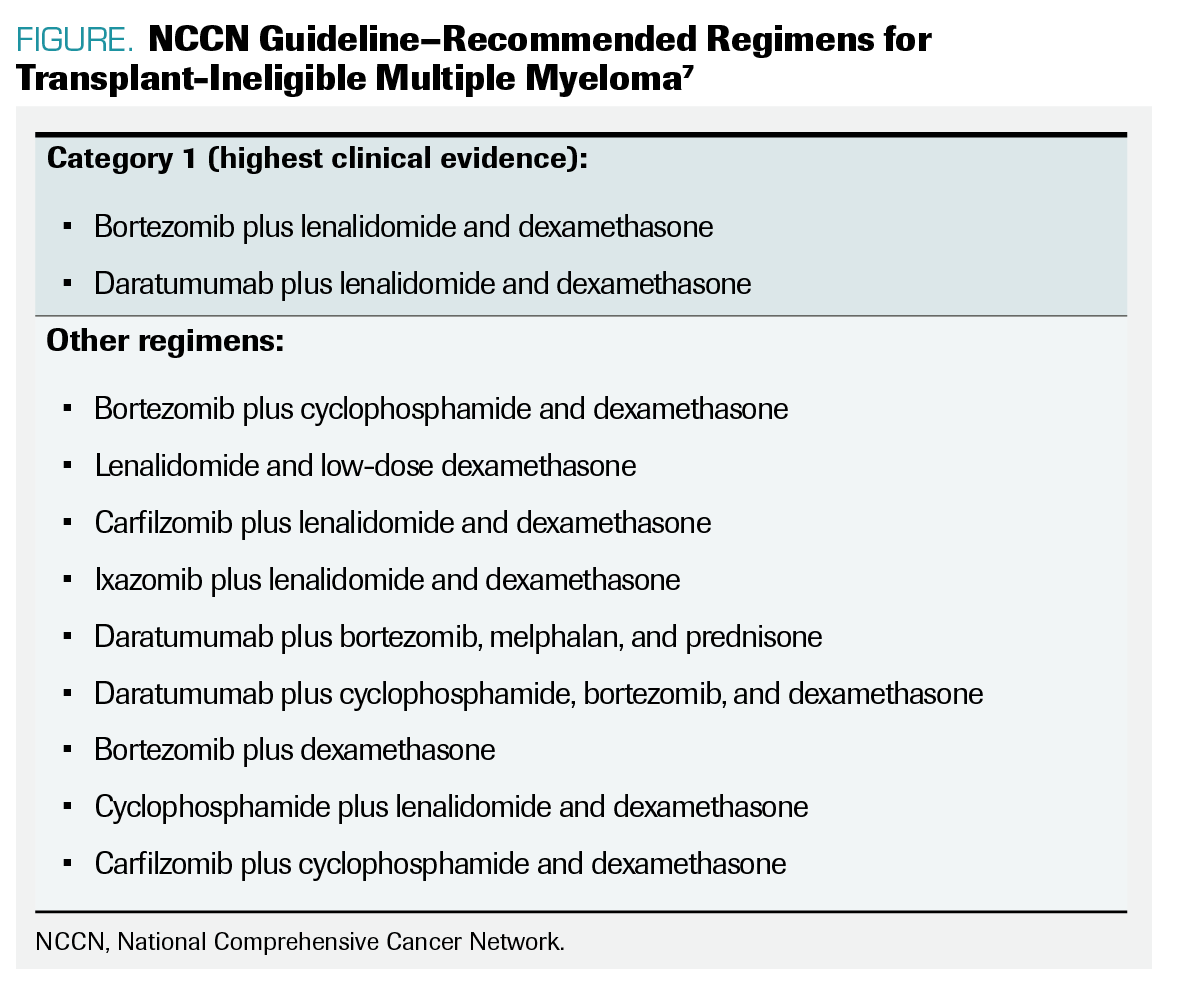
FIGURE. NCCN Guideline–Recommended Regimens for Transplant-Ineligible Multiple Myeloma
In the MAIA trial (NCT02252172) of daratumumab (Darzalex) plus Rd in patients with transplant-ineligible myeloma, results showed that adding daratumumab to Rd was superior to outcomes with Rd alone.6 All 3 agents were continued until disease progression or unacceptable toxicity was experienced.
Gasparetto said that to maintain tolerability, it may be necessary to deescalate dexamethasone at a certain point. “After a while, you have to deescalate some of the therapy.” she said. “MAIA is a deescalation by protocol because the daratumumab [is eventually administered] monthly, and so you may choose to discontinue the dexamethasone and continue the lenalidomide, so I do that.”
Using protocols with 3 or more drugs can make it easier to adjust therapy according to patient needs. “I think we feel emboldened to be able to make some adjustments more readily when the patient’s quality of life is suffering,” Voorhees said. “When you have somebody on these 3 drugs, and they have terrible fatigue and diarrhea from the lenalidomide, you’ll feel much more comfortable stripping that away if they are still on the daratumumab.”
The National Comprehensive Cancer Network guidelines outline therapeutic options for transplant-ineligible multiple myeloma based on clinical evidence (FIGURE).7
Patient Treatment
Panelists then considered 2 cases of patients with multiple myeloma:
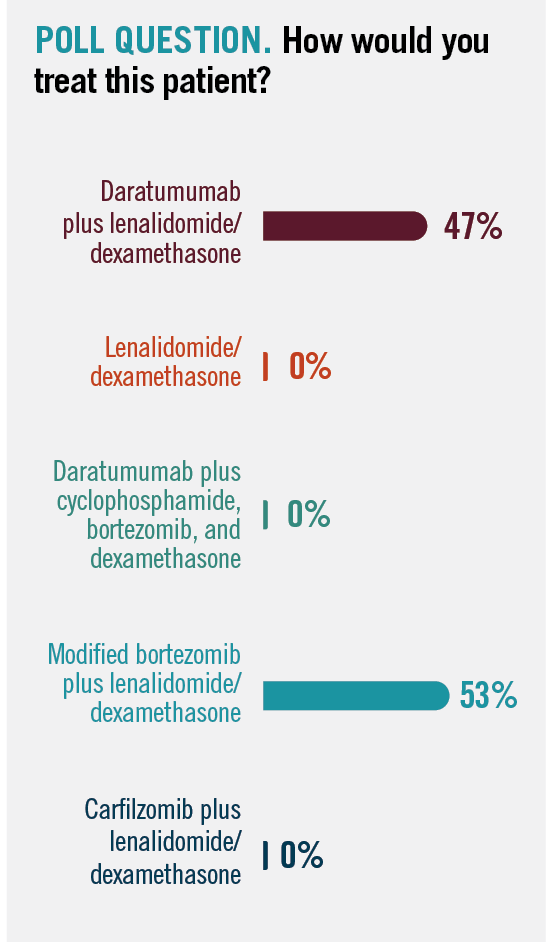
Audience members who participated in the presentations voted on which treatment regimen they would use to treat this patient, and all participants chose to give either daratumumab plus Rd or modified bortezomib plus Rd (Poll question).
Regarding maintenance therapy, Landgren said he typically switches patients over to a single-drug regimen. “For patients in this older category, safety and toxicity are probably [among] my main factors [for determining continuation of therapy],” he said.
In another case, an elderly patient with transplant-ineligible disease requested to stop therapy despite experiencing good tolerability and achieving a good response to therapy.
In this situation, Gasparetto said she would try to understand the patient’s reasons for wanting to stop therapy. “It’s clear that we have lots of data proving that continuation is important, so stopping at 6 cycles with a VGPR [means we will be likely] to run in trouble relatively soon,” she pointed out. “We want to keep the response longer.”
With this, she referred back to the discussion about therapy deescalation to get the patient to agree to continue treatment. “On the MAIA study, several patients came off the lenalidomide but were able to sustain the daratumumab monthly and continued to maintain the response for several months,” Gasparetto noted.
POLL QUESTION. How would you treat this patient?
REFERENCES
1. Multiple myeloma: statistics. Cancer.net. August 2021. Accessed November 10, 2021. https://bit.ly/3CbtiSd
2. Nandakumar B, Binder M, Dispenzieri A, et al. Continued improvement in survival in multiple myeloma (MM) including high-risk patients. J Clin Oncol. 2019;37(suppl 15):abstr 8039. doi:10.1200/JCO.2019.37.15_suppl.8039
3. SEER explorer: myeloma: recent trends in SEER age-adjusted incidence rates, 2000-2018. National Cancer Institute / Surveillance, Epidemiology, and End Results Program. Accessed November 11, 2021. https://bit.ly/3F8dYYB
4. Mina R, Bringhen S, Wildes TM, Zweegman S, Rosko AE. Approach to the older adult with multiple myeloma. Am Soc Clin Oncol Educ Book. 2019;39:500-518. doi:10.1200/EDBK_239067
5. Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527. doi:10.1016/S0140-6736(16)31594-X
6. Facon T, Kumar S, Plesner T, et al; MAIA Trial Investigators. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104-2115. doi:10.1056/NEJMoa1817249
7. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 3.2021. Accessed November 11, 2021. https://bit.ly/3D7ECQV
EP: 1.Challenges in Treating Transplant-Ineligible Multiple Myeloma
EP: 2.Multiple Myeloma: Transplant Ineligibility and Goals of Therapy
EP: 3.Transplant-Ineligible Multiple Myeloma: Assessing Response to Therapy
EP: 4.Transplant-Ineligible MM: Treatment After Adequate Response to Therapy
EP: 5.Treatment Options for First-Line Therapy in Transplant-Ineligible MM
EP: 6.Patient Case #1: A 75-Year-Old Woman With Newly Diagnosed MM
EP: 7.Patient Case #2: A 77-Year-Old Man With Newly Diagnosed MM
EP: 8.Future Directions in Multiple Myeloma Care: Improvements and Unmet Needs
EP: 9.Recap: Optimizing Therapy in Frontline Multiple Myeloma Not Eligible for Transplant

Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.