Recap: Prognostic Potential of ctDNA for Oligometastatic Colorectal Cancer
Richard Kim, MD, and Lilliana Bustamante, MD, discuss the potnetial for using ctDNA to treat patients with oligometastatic colorectal cancer.
With approximately 50% of patients with oligometastatic colorectal cancer (CRC) relapsing following surgical resection, effective prognostic biomarkers that can identify residual disease are needed in this patient population.1,2
Similarly, the efficacy of postoperative systemic treatment is currently limited for patients with stage IV CRC, and patients may benefit greatly from a reliable biomarker to identify minimal residual disease (MRD).
Richard Kim, MD, and Liliana Bustamante, MD, discussed the potential of circulating tumor DNA (ctDNA) as an effective biomarker for patients with CRC in a recent Between the Lines program hosted by CancerNetwork®.
“As of right now, the only biomarker that we typically use in the setting is CEA [carcinoembryonic antigen], which we typically check before and after surgery,” explained Kim, who is service chief of Medical Gastrointestinal Oncology and senior member in the Gastrointestinal Oncology Department at the Moffitt Cancer Center in Tampa, Florida. “Now we’re trying to figure out the prognostic role of the circulating tumor DNA in this setting, which is a very novel idea.”
“Circulating tumor DNA is something that we can easily get that could help guide us in knowing how the patient is going to do and hopefully guide our treatments going forward,” Bustamante, a hematologist and medical oncologist at Florida Cancer Specialists in Fort Myers, Florida, added.
During the discussion, Kim and Bustamante examined the use of ctDNA for MRD detection in the context of the PREDATOR trial, which featured patients with metastatic CRC who underwent metastatic resection with curative intent. After resection, 39.2% of patients received systemic therapy.3
Patient Demographics and ctDNA Results
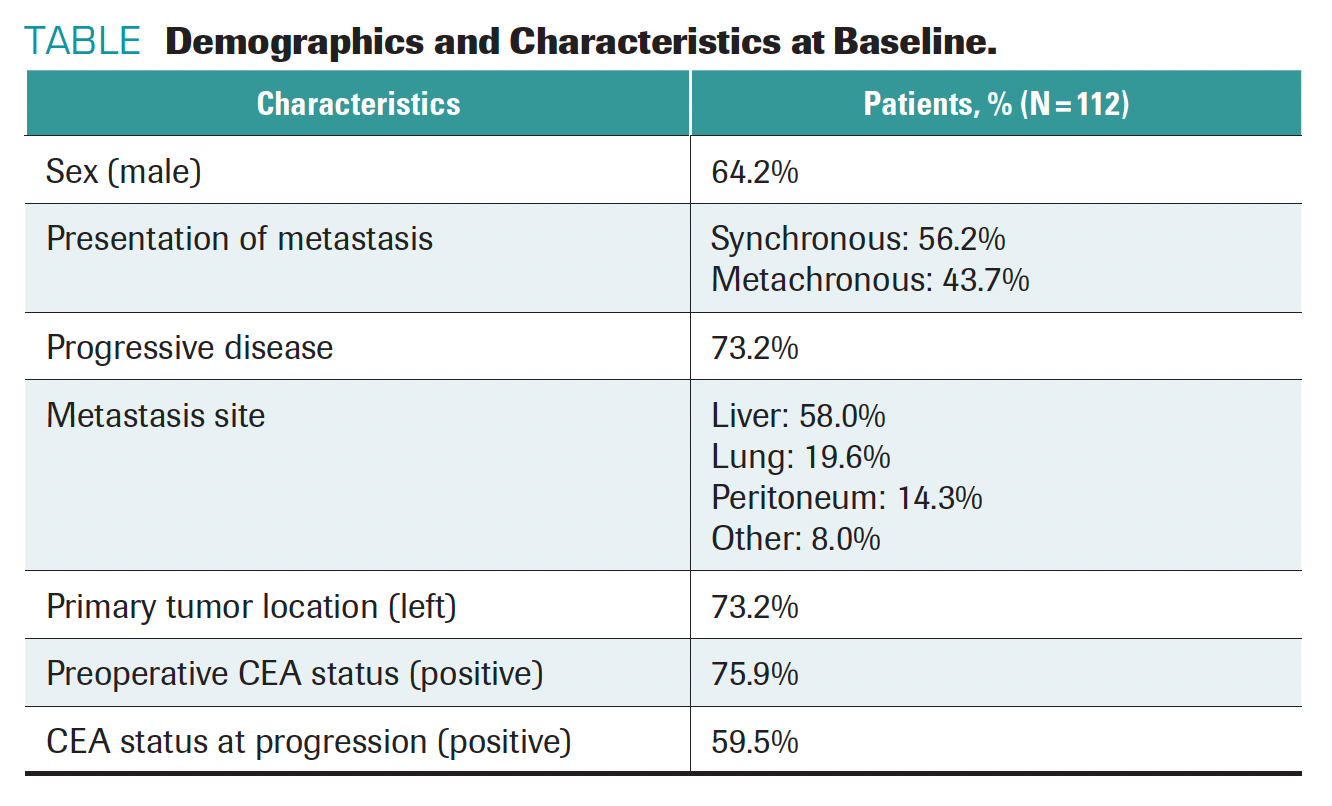
A total of 112 patients with metastatic CRC who underwent metastatic resection were enrolled, with ctDNA drawn after surgery and at the time of radiologic evidence of progressive disease or last follow-up. In total, 82 patients experienced disease progression.
Analysis of ctDNA was performed on 192 samples from this cohort, with a
median follow-up of 10.7 months (range, 0.9-53.8 months). The median age of patients was 60.1 years, and disease progression was observed in 73.2% of patients (TABLE).
TABLE. Demographics and Characteristics at Baseline
Patients with MRD positivity characterized 54.4% of the total cohort, or 61 patients. Of these, 59 patients (96.7%) had disease progression at the data cutoff (HR, 5.8; 95% CI, 3.5-9.7; P < .001). MRD positivity at 2 time points, baseline and follow-up, was a strong indicator of outcome, with those having 2 ctDNA-positive tests having a high likelihood of disease progression without systemic therapy (FIGURE).
FIGURE. ctDNA Status at Baseline and Follow-up in Patients With Disease Progression
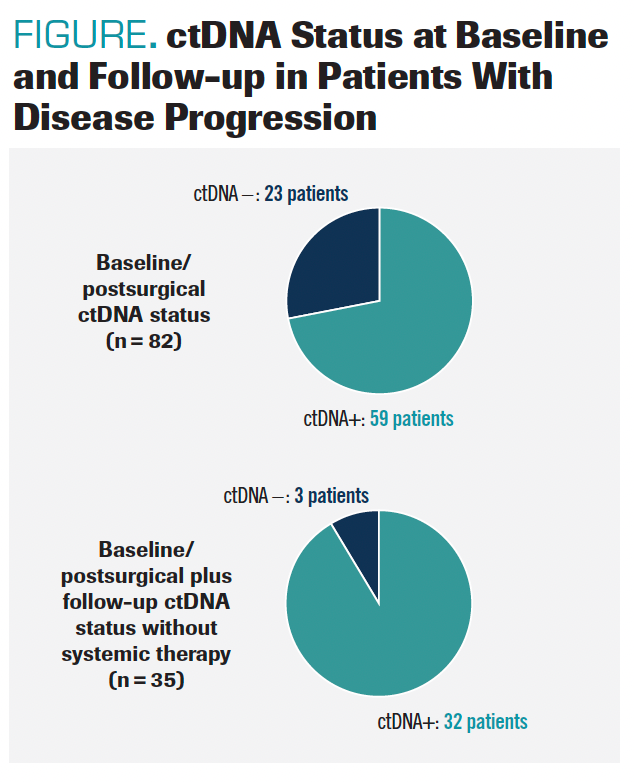
“There’s a clear separation telling us that [ct]DNA positivity is associated with a poor prognosis in terms of disease-free survival [DFS] and overall survival [OS] where patients who are circulating tumor DNA–negative tend to do well and their disease-free survival is much longer than the patients who are circulating tumor DNA–positive,” Kim explained.
He continued by stating that the sensitivity of baseline ctDNA was just above 70% but correlating findings from baseline ctDNA with that collected at 6 or 8 weeks of follow-up resulted in the sensitivity increasing to over 80%.
“If you look at the correlation between a one-time baseline blood draw and try to [connect] that with [survival outcomes], there’s a clear correlation between positive circulating tumor DNA and the worst prognosis in terms of the disease-free survival and overall survival. The patients who are negative for circulating tumor DNA at baseline tend to do much better,” Kim said.
MRD positivity was associated with inferior OS vs MRD negativity (HR, 16.0; 95% CI, 3.9-68.0; P < .001). At the data cutoff, 49 of 51 patients (96%) who were MRD negative were alive vs only about half of those who were MRD positive (32 of 61; 52.4%).
“The biggest takeaway is that the only variable that showed a statistically significant correlation with disease-free survival was the circulating tumor DNA,” Bustamante explained.
For patients with ctDNA negativity at both baseline and follow-up blood draws, the OS rate was 100% at 50 months of follow-up. Expanding on this, Kim explained “If you have a multiple draw, the sensitivity tends to go up as well, clearly telling us that there is a prognostic role of drawing tumor DNA in this setting in patients who undergo surgery [and do] not get chemotherapy in this study.”
Conclusions and Future Directions
A main takeaway from this study is the prognostic potential of ctDNA for detecting progression in CRC, especially over 2 time points postoperatively, according to Bustamante.
Bustamante emphasized that 2 negative tests at different points provides some reassurance that clinicians may safely observe their patients instead of treating them systemically. It also provides comfort to patients because there are data linking positive survival and testing negative twice by ctDNA.
Kim explained that questions remain regarding ctDNA, specifically around its predictive ability. “The dilemma that we will be facing after surgery if the circulating tumor DNA is positive is what to do at that time, understanding that there is no radiographic evidence of disease,” he said.
“Regardless,” Kim concluded, “this is a prognostic biomarker that we can use in conjunction with the radiographic imaging and CEA as well to help better guide patients [regarding treatment and monitoring] after surgery.”
“This paper showed that CEA levels do not have good sensitivity in predicting who’s going to progress or not as compared to this test. In this case, ctDNA will help us predict patients’ prognosis or risk of recurrence and hopefully help guide how we follow them and how we treat them,” Bustamante explained.
References
1. Lin J, Peng J, Zhao Y, et al. Early recurrence in patients undergoing curative resection of colorectal liver oligometastases: identification of its clinical characteristics, risk factors, and prognosis. J Cancer Res Clin Oncol. 2018;144(2):359-369. doi:10.1007/s00432-017-2538-8
2. Pan Z, Peng J, Lin J, et al. Is there a survival benefit from adjuvant chemotherapy for patients with liver oligometastases from colorectal cancer after curative resection? Cancer Commun (Lond). 2018;38(1):29. doi:10.1186/s40880-018-0298-8
3. Loupakis F, Sharma S, Derouazi M, et al. Detection of molecular residual disease using personalized circulating tumor DNA assay in patients with colorectal cancer undergoing resection of metastases. JCO Precis Oncol. 2021;5(5):PO.21.00101. doi:10.1200/PO.21.00101
EP: 1.Presentation: ctDNA Assays For Patients With CRC Undergoing Resection of Metastases
EP: 2.Key Takeaways: ctDNA Assays For Patients With CRC Undergoing Resection of Metastases
EP: 3.ctDNA as a Biomarker in CRC
EP: 4.ctDNA Assays in CRC: Interpreting Clinical Findings
EP: 5.ctDNA Assays in CRC: Impact on Clinical Practice
EP: 6.Next Steps in Using ctDNA as a Biomarker in CRC
EP: 7.Recap: Prognostic Potential of ctDNA for Oligometastatic Colorectal Cancer
EP: 8.Between the Lines™ Podcast: ctDNA as a Biomarker of Progression in Colorectal Cancer
