Receptor Polymorphisms and Gene Clusters May Help Identify Individual Patients Most Likely to Benefit From Immunotherapy
This special supplement to Oncology NewsInternational includes updated results ofstudies with anti-CD20 therapy and othertargeted therapies in the treatment oflymphomas, chronic lymphocytic leukemia,and immune thrombocytopenic purpura. Theresults were presented at the American Societyof Hematology 44th Annual Meeting inPhiladelphia, December 6 to 10, 2002.City Hall in Philadelphia, Pennsylvania
PHILADELPHIA-Developingmethods to determine which patientswill benefit most from immunotherapywill allow physicians to bettertailor therapy for individual patients.Such methods for predicting responseto treatment will be particularly usefulin indolent relapsing diseases suchas follicular lymphoma, and may reducethe number of treatment regimensthat patients receive during thecourse of their disease. Two presentationsby investigators at Stanford Universityin California identified methodsthat may make it possible topredict patient responses to antibodytherapy.[1,2]Fc Gamma RIIIaPolymorphismsWen-Kai Weng, MD, PhD, RonaldLevy, MD, and colleagues investigatedimmunoglobulin G Fc receptor Fcgamma RIIIa polymorphisms inpatients with relapsed follicular non-Hodgkin's lymphoma treated withrituximab (Rituxan) (ASH abstract1368).[1] Rituximab antitumor effectsare mediated, in part, by antibody-dependent cellular cytotoxicity.Recently, the Fc gamma RIIIa position158 valine/valine genotype wasdemonstrated to correlate withimproved clinical response to rituximabcompared with valine/phenylalanineor phenylalanine/phenylalanine(phenylalanine carriers) inpatients with previously untreatedfollicular lymphoma.[3] The StanfordUniversity team sought to testthis hypothesis in patients withrelapsed follicular lymphoma.
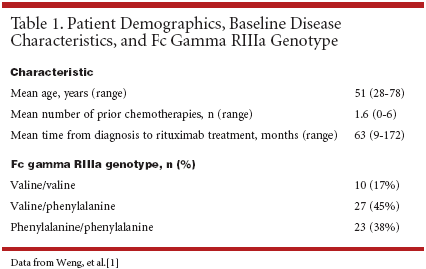
Patient demographics and baselinecharacteristics are detailed inTable 1.[1] Dr. Levy reported that ananalysis of 60 patients revealed that Fcgamma RIIIa position 158 genotypedid not influence rituximab responserate 1 to 3 months after treatment inpatients with previously treated follicularlymphoma (see Table 2).[1]
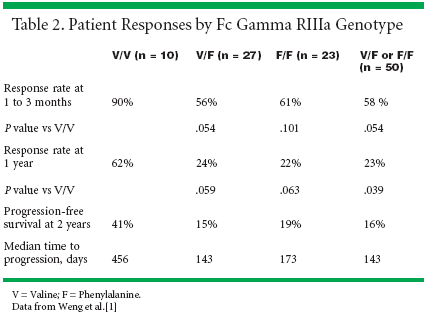
However, 12 months after rituximabtreatment, patients with position 158valine/valine genotype had a higherresponse rate (62%) than the phenylalaninecarriers (23%; P = .039). Furthermore,the progression-free survivalat 2 years was 41% for patientswith position 158 valine/valine genotypevs 16% for phenylalanine carriers,although the difference did notachieve statistical significance. Fromthese results, the investigators confirmedthe predictive value of Fc gammaRIIIa polymorphisms in patientswith previously treated follicular lymphomaand suggested that the efficacyof rituximab may be improved if itsbinding affinity for Fc gamma RIIIacan be enhanced.Gene Expression PatternsIn addition to antibody-dependentcellular cytotoxicity, other proposedmechanisms of action for rituximabactivity in CD20-expressing lymphomasare complement-mediated lysisand induction of apoptosis. In an effortto further define the mechanismof action of rituximab in patients withfollicular lymphoma and to develop amethod for predicting responses torituximab, Sean Bohen, MD, PhD,and colleagues examined the expressionof more than 20,000 genes bymicroarray (ASH abstract 1222).[2,4]An analysis of 16 patients revealedtwo clusters of gene expression patterns:patients who did not respondto rituximab displayed expression patternsmore similar to normal tonsiland spleen lymphoid tissue, whereaspatients responded to rituximab clusteredin a second group (P = .002).Specifically, the genes identified inthe nonresponders were those thatplay a role in cellular immunity.The authors speculated that differencesin these genes suggest thatthe nonresponders may have a reducedcapacity for eliciting an antilymphomaimmune response. Furtheranalysis of 24 patient samplesidentified more than 100 genes expressedat significantly different levelsin rituximab responders vs nonresponders.More than one-third ofthese genes were involved in the cellularimmune response. These genesand other cellular immunity geneswere then compared with thoseknown to be up-regulated by macrophagesactivated in vitro by bacterialpathogens. Once again, rituximabresponders and nonresponders clusteredinto groups based on the expressionof 136 macrophage activationgenes (P < .013).These two analyses of receptorpolymorphisms and gene clusters thatcorrelate with responses with rituximabsuggest that screening beforetherapy would allow the selection ofpatients who are most likely to benefitfrom immunotherapy. Furthermore,these analyses provide importantnew information regarding themechanisms of action of antibodytherapy and identify potential targetsfor antibody modification that mayimprove the number and intensity ofpatient responses to antibody therapy.
References:
1.
Weng WK, Levy R. Analysis ofIgG Fc receptor Fc gamma IIIa polymorphismin relapsed follicular non-Hodgkin’s lymphoma patients treatedwith rituximab (abstract 1368).Blood 100:353a, 2002.
2.
Bohen SP, Troyanskaya O, AlterO, et al: Predicting rituximab responseof follicular lymphoma using cDNAmicroarray analysis (abstract 1222).Blood 100:316a, 2002.
3.
Cartron G, Dacheux L, Salles G,et al: Therapeutic activity of humanizedanti-CD20 monoclonal antibodyand polymorphism in IgG Fc receptorFc gamma RIIIa gene. Blood99:754-758, 2002.
4.
Bohen SP, Troyanskaya OG,Alter O, et al: Variations in gene expressionpatterns in follicular lymphomaand the response to rituximab.Proc Natl Acad Sci USA100:1926-1930, 2003.
Oncology Peer Review On-The-Go: Minority Treatment Disparities and Clinical Trial Enrollment
July 6th 2020The first episode of CancerNetwork's podcast Oncology Peer Review On-The-Go explores disparities in cancer care treatment among minorities and the significance of a representative sample in clinical trials.