Recognizing and Managing Side Effects Associated With Novel Targeted Therapies:
Nursing management of patients with advanced malignancies presents a formidable challenge. In addition to the discomfort and debilitation these diseases can cause, side effects of traditional treatment modalities such as surgery, chemotherapy, and radiation may lead to severe and sometimes fatal sequelae. New targeted therapies promise an effective treatment with more easily tolerated and managed side effects. Basic understanding of the drugs' mechanism of action contributes to the successful management of the toxicities that can be manifested. Effective patient education results in improved compliance with treatment regimens and potentially improved clinical outcomes. Nursing intervention remains a vital component in the successful use of these novel agents.
Nursing management of patients with advanced malignancies presents a formidable challenge. In addition to the discomfort and debilitation these diseases can cause, side effects of traditional treatment modalities such as surgery, chemotherapy, and radiation may lead to severe and sometimes fatal sequelae. New targeted therapies promise an effective treatment with more easily tolerated and managed side effects. Basic understanding of the drugs' mechanism of action contributes to the successful management of the toxicities that can be manifested. Effective patient education results in improved compliance with treatment regimens and potentially improved clinical outcomes. Nursing intervention remains a vital component in the successful use of these novel agents.
Nursing management of patients with advanced malignancies presents a formidable challenge. In addition to the discomfort and debilitation these diseases can cause, side effects of traditional treatment modalities such as surgery, chemotherapy, and radiation may lead to severe and sometimes fatal sequelae. New targeted therapies promise an effective treatment with more easily tolerated and managed side effects. However, nursing management remains a vital component in the successful use of these novel agents.
New Targeted Agents
The US Food and Drug Administration (FDA) has granted approval to the following agents that target specific areas of two important molecular pathways in solid tumors-the HER family (EGFR/HER1 and HER2) and the VEGF family: erlotinib (Tarceva), gefitinib (Iressa), sorafenib (Nexavar), sunitinib (Sutent), cetuximab (Erbitux), trastuzumab (Herceptin), bevacizumab (Avastin), and panitumumab (Vectibix). Lapatinib (Tykerb) is expected to receive approval within the next few months. Clinical trials continue for ZD6474 (Zactima) in various solid tumors.
Erlotinib, gefitinib, cetuximab, trastuzumab, bevacizumab, and panitumumab are all agents with single targets. Sorafenib, sunitinib, lapatinib, and ZD6474 have more than one molecular target and are considered multitargeted agents.
Erlotinib and gefitinib were originally approved for the treatment of advanced non-small-cell lung cancer, but use of gefitinib was subsequently restricted to patients for whom the treatment had previously proved effective.[1] Sorafenib has been approved for treatment of advanced renal cancer[2] and lapatinib is expected to receive approval for treatment of advanced breast cancer.[3]
Sunitinib has recently been FDA-approved for the treatment of patients with gastrointestinal stromal tumors and advanced kidney cancer-two tumor types that have been very difficult to treat.[4,5]
The monoclonal antibody (MoAb) cetuximab is approved for use in advanced colorectal and head and neck cancers,[6] and panitumumab recently received approval for treatment of advanced colorectal cancers.[7] Trastuzumab, another MoAb, is approved for HER2-positive breast cancer and has been tested in lung and other solid tumors.[8] Bevacizumab is currently approved for use in metastatic colorectal cancers, and has recently received approval for use as first-line therapy for nonsquamous non-small-cell lung cancer.[9] All of these agents continue to be tested in other solid tumors.
Mechanism of Action
The mechanism of action for the epidermal growth factor receptor (EGFR) inhibitors is the blockade of the activation of the tyrosine kinase (TK) signal transduction pathway that is a required communication activity of both normal and malignant cells. The interruption of this pathway, which is aberrantly activated or overexpressed in malignant cells, is thought to interfere with the cellular functions of growth, proliferation, invasion or metastasis, angiogenesis, and survival or apoptosis and thus leads to control or death of the cancer cells. The same mechanism may interfere with the normal functions of epithelial cells and produce the constellation of toxicities associated with the EGFR inhibitors.[10]
The orally administered small-molecule TK inhibitors erlotinib and gefitinib interrupt the signal transduction pathways inside the cell. The MoAbs cetuximab, trastuzumab, bevacizumab, and panitumumab, administered intravenously, interrupt the pathways on the cell surface.[11,12] Sorafenib, sunitinib, ZD6474, and lapatinib, which are orally administered, are multikinase inhibitors that inhibit more than one signal transduction pathway and have their site of activity within the cell.[13]
Adverse Experiences Associated With novel Targeted therapies
Each of these drugs has a unique pattern of action and resulting side effects or toxicities. In the following sections, we will discuss in detail some of the more prevalent side effects that oncology nurses may encounter in their practice.
Rash and Diarrhea Associated With EGFR Inhibitors
Skin rash and diarrhea are the most common side effects of all EGFR inhibitors. (Dermatologic toxicities will be discussed in detail in the article by O'Keeffe et al found on page 25 of this supplement.).Other commonly experienced toxicities include dry skin and eyes, itching, paronychial inflammation and fissuring, occasional nausea and vomiting, and asthenia or malaise. Hypomagnesemia (also associated with cetuximab and panitumumab[6,7]), hypercalcemia, and hypokalemia have recently been reported as well. Less common but potentially very dangerous are infusion reactions associated with monoclonal antibodies (less than 3%) and interstitial lung disease (less than 1%).[2]
Diarrhea has been the major dose-limiting toxicity in clinical trials of erlotinib and gefitinib, although it is less commonly seen with cetuximab. Diarrhea was observed in approximately 50% of patients in early clinical trials (54% with erlotinib and 58% with gefitinib). The diarrhea is usually classified as grade 1 or 2, with rare grades 3 and 4 being reported. It has been described as loose, watery stools without the presence of mucus or blood. In most instances, the diarrhea occurs within the first three weeks of treatment.[14,15]
Nursing care of patients experiencing diarrhea associated with EGFR inhibitors focuses on assessment, accurate grading (Table 1), and early intervention. Patients should be thoroughly assessed for normal bowel habits before initiation of therapy so that changes can be more easily evaluated. Information about the likely incidence and severity of diarrhea should be provided to patients and their families. As treatment progresses, nurses should ask specific questions to ascertain changes in frequency or character of stools. Use of a consistent grading scale is essential in the clinician's practice, and the scale and its implications should be shared with the patient. The National Cancer Institute's Common Toxicity Criteria provide an excellent tool for this purpose, enabling patient assessment by telephone.[16]
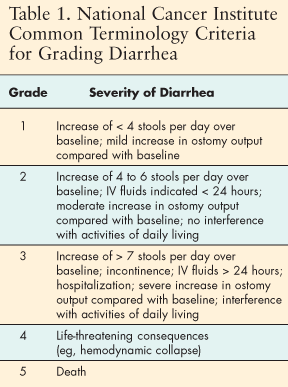
Over-the-counter (OTC) medications such as loperamide have proved effective in control of grade 1 or 2 diarrhea, and patients should have this medication on hand before beginning treatment with an EGFR inhibitor. Instruct patients to report diarrhea with the first loose stool and to begin treatment immediately. Patients are given written instruction for use of OTC loperamide at doses exceeding package instructions and continuing as necessary. In our practice we often provide preprinted adhesive labels to apply to the OTC Immodium with specific dosing instructions. If OTC medications are not effective to control the diarrhea, prescription medications such as Lomotil or Sandostatin may be started. Patient education concerning standard measures for diarrhea, including increased fluid intake, dietary modifications, and special care for excoriated perianal tissues should be initiated.
If these measures do not control the diarrhea, and it progresses to grade 3 or 4, more aggressive treatment is required. At grade 3, a temporary dose reduction or interruption should be considered. For example, the dose of erlotinib can be reduced in 50-mg increments. Dose reductions are not available for gefitinib, but each of these drugs can be interrupted for up to 14 days. This reduction or interruption is often sufficient to control the diarrhea, and patients may then continue treatment. Rarely, IV hydration may be required if the diarrhea is not effectively controlled.
As with any diarrhea, thorough evaluation is essential to rule out other possible causes. If an underlying infection such as Clostridium difficile is identified, adequate medical treatment should be initiated. Patients with persistent diarrhea who do not respond to other therapies usually receive antibiotics. Follow-up is necessary to evaluate efficacy and plan further therapy.[17-19]
Side Effects Associated With Sorafenib
The most common adverse events associated with sorafenib are similar in scope and incidence to the other tyrosine kinase inhibitors. However, the drug is also known to cause hand-foot syndrome in approximately 30% to 35% of treated patients. About 6% of patients experiencing hand-foot syndrome have a grade 3/4 toxicity.[2]
The dermatologic side effect of hand-foot syndrome associated with sorafenib differs from the dermatologic effects of EGFR inhibitors. Hand-foot skin reactions usually begin as a mild tingling or pain on the palms or soles of the feet within the first 6 weeks after starting the medication. This can progress to erythema and cracking or peeling of the skin that may interfere with normal activities. Some patients find ambulation difficult and painful. Sorafenib is stopped while the skin heals and symptoms abate. The drug can then be restarted at a lower dose; ie, 400 mg once daily with a further reduction to 400 mg every other day.[2]
Side Effects Associated With Sunitinib
The most commonly reported sunitinib-related side effects included diarrhea, skin discoloration, mouth irritation, weakness, and altered taste. Patients treated with sunitinib also experienced fatigue, high blood pressure, bleeding, swelling, and taste disturbance. Hypothyroidism has also been observed.[4,5]
Cardiovascular Toxicities Seen With VEGF Inhibitors and Multitargeted Agents
Hypertension has been identified as a common side effect of both single-targeted and multitargeted drugs that inhibit the vascular endothelial growth factor (VEGF) pathways. Hypertension has been associated with bevacizumab, sorafenib, and especially with ZD6474. It is usually described as a systolic pressure greater than 140 mm/Hg and a diastolic pressure greater than 90 mm/Hg. Hypertension can be aggressively treated by a traditional medical approach, with dose reductions necessary only if these approaches fail or the patient experiences sustained symptoms.[2,9,20]
Patients being treated with these agents should be instructed in the use of home blood pressure monitors and informed of the criteria for calling the clinician. ACE inhibitors and beta blockers have been successfully used to control the hypertension associated with these drugs. If the drugs are held or discontinued, careful blood pressure monitoring is indicated to prevent development of hypotension.
In addition to hypertension, ZD6474 has been associated with the development of asymptomatic QTc prolongation. This delay in cardiac repolarization can lead to torsades de pointes, a potentially fatal variation of ventricular tachycardia. For this reason, other medications that affect the QTc interval are not coadministered with ZD6474, nor is it prescribed for patients with baseline QTc prolongation, and EKGs are closely monitored for changes from baseline. Serum magnesium and electrolytes are checked regularly, as deficiencies in these levels can contribute to the risk of QTc prolongation. Patients should be provided with a comprehensive list of medications to avoid while taking ZD6474.[20]
Trastuzumab has been associated with multiple cardiac toxicities including dyspnea, peripheral edema, and congestive heart failure that can be severe enough to cause mural thrombosis and potentially death. Candidates for treatment with trastuzumab should be carefully assessed at baseline for any cardiac dysfunction. Patients undergoing treatment should undergo frequent monitoring for deteriorating cardiac function. Discontinuation should be strongly considered in patients who develop a clinically significant decrease in ejection fraction during therapy. Combination therapy with anthracyclines and cyclophosphamide increases the risk of cardiac dysfunction.[8] Recent information released by the manufacturer indicated that arterial thrombotic events have been associated with bevacizumab.[9]
Infusion Reactions Associated With Monoclonal Antibodies
Infusion reactions are an uncommon but potentially lethal toxicity related to administration of MoAbs, including cetuximab, bevacizumab, panitumumab, and trastuzumab. Because cetuximab is a chimeric MoAb (mouse/human), reactions may occur more frequently than with humanized MoAbs like bevacizumab or trastuzumab or with fully human MoAbs such as panitumumab. However, the occurrence of severe infusion reactions even with cetuximab are uncommon, occurring about 3% of the time.
Infusion reactions can range from mild or moderate, characterized by flushing, rash, fever, and itching, to full-blown anaphylaxis. Most reactions (90%) are associated with the first infusion, but they can occur even after multiple infusions. Trastuzumab is a humanized monoclonal antibody but may still cause infusion reactons up to 24 hours after dosing.[8] Infusion reactions are reported in approximately 40% of treated patients. These reactions can be as mild as chills and fever. Treatment with acetaminophen, diphenhydramine, and/or meperidine is usually effective.[8] Panitumumab (Vectibix) has been associated with infusion reactions in only about 1% of treated patients. Severe infusion reactions were identified by reports of anaphylactic reaction, bronchospasm, fever, chills, and hypotension. Fatal infusion reactions have not been reported with panitumumab.[7]
The following are the most common signs and symptoms of anaphylaxis seen with grade 3 or 4 reactions:
• Respiratory changes: Rapid onset of airway obstruction such as dysphonia, stridor, cough, wheezing, or dyspnea
• Altered mental status: Lightheadedness, headache, seizures, loss of consciousness (may be an indicator of hypoxia)
• Cardiac changes: Hypotension with or without syncope or cardiac arrhythmias and shock
• Skin changes: Localized or diffuse erythema, urticaria, pruritus, and angioedema
• Gastrointestinal changes: Nausea, vomiting, or diarrhea
All of these symptoms may present in a milder form, as grade 1 or 2. Anaphylaxis occurs as part of a continuum, and early recognition of symptoms and prompt intervention is imperative to prevent more severe and potentially fatal reactions (Tables 2 and 3).[21]
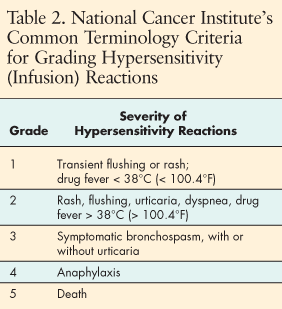
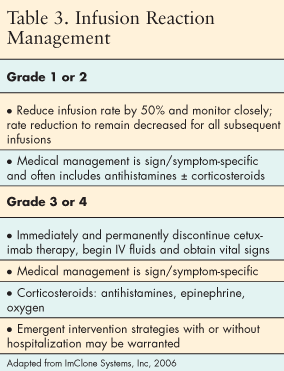
Nursing management of infusion reactions associated with MoAbs begins with careful preparation for the infusion. Appropriate medical therapy should be available to prevent or manage any reaction. Proper premedication as ordered by the physician should be administered before the infusion begins. Standard operating procedures and institutional guidelines should be in place for immediate treatment of severe or life-threatening emergencies. Medical therapy should be available to manage reactions. Epinephrine, corticosteroids, IV antihistamines, bronchodilators, and oxygen, along with a well-stocked and maintained crash cart, should be in place and immediately available.
The package insert for cetuximab[6] recommends the following intervention for infusion reactions:
"If a grade 1 or 2 hypersensitivity reaction occurs, the first response is to stop the infusion. Diphenhydramine may be repeated and the use of steroids considered for delayed reaction. If all symptoms resolve, the patient may be rechallenged by restarting the infusion at 50% decrease in rate. If no further symptoms develop, the infusion may continue. The rate of cetuximab should never be increased over the 50% reduction for future infusions. If symptoms return, the infusion should be stopped and treatment with cetuximab discontinued. It is recommended that epinephrine be used at the first sign of anaphylaxis."
The National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network has also published a useful algorithm for the treatment of anaphylaxis, which is available at http://www.acaai.org/Member/SAFE_Diagnosis_Criteria.htm.
Patients should be monitored for at least 1 hour following each infusion of cetuximab. A patient who has a severe or life-threatening reaction should never be rechallenged with cetuximab.[6]
Interstitial Lung Disease
Interstitial lung disease is an uncommon but potentially life-threatening toxicity associated with the use of EGFR inhibitors (cetuximab < 0.5%, erlotinib 0.6%, and gefitinib 1%).[1,6,22] This toxicity, like infusion reactions, requires astute assessment and immediate intervention. It is often associated with an abrupt onset. A sudden exacerbation of pulmonary symptoms or onset of new symptoms such as dyspnea, worsening cough, or hemoptysis requires prompt action. The EGFR inhibitor should be interrupted immediately, a diagnostic evaluation undertaken to determine the cause of the new or worsening symptoms, and, if a diagnosis of interstitial lung disease is confirmed, the treatment with EGFR inhibitors should be permanently discontinued. Patients must then be referred to a pulmonary specialist to manage the condition.
Pulmonary Fibrosis
Pulmonary fibrosis is a rare occurrence that has been associated with the use of panitumumab in clinical trials. Less than 1% of patients treated (2/1,467) exhibitied this toxicity. One of these patients had preexisting idiopathic pulmonary fibrosis and was treated in combination with chemotherapy. The patient died of worsening pulmonary fibrosis after 4 doses of panitumumab. The second case developed symptoms of pulmonary fibrosis, including cough, wheezing, exertional dyspnea, and CT evidence of pulmonary fibrosis while being treated with panitumumab as a single agent. Current clinical trials exclude patients with history or evidence of interstitial pneumonitis or pulmonary fibrosis. Panitumumab should be permanently discontinued in patients developing interstitial lung disease, pneumonitis, or lung infiltrates.[7]
Drug and Food Interactions
Drug-drug and drug-food interactions are also possible with erlotinib, gefitinib, sorafenib, ZD6474, and lapatinib. The CYP3A4 enzyme is known to metabolize the drugs through the liver, and potent inducers or inhibitors of the pathway should be avoided for concomitant use. A specific list of drugs and foods to be avoided must be provided to patients. Use of alternative therapies should also be closely monitored; some substances, such as St. John's wort, should not be taken.
Nursing Interventions
The following are key elements of nursing management for toxicities associated with targeted agents:
• Basic understanding of the mechanism of action on cells
• Knowledge of dosing and administration guidelines
• Familiarity with the toxicities associated with their use
• Strategies for monitoring patients' progress and assessing and grading toxicities
• Evidence-based guidelines for treatment of toxicities
• Plans and materials for patient education
• Tools to help patients maximize compliance with dosing and to prevent or modulate toxicities
Inherent in nursing is the responsibility to provide sufficient educational instruction, information, and materials to enable patients to perform adequate self-care. Nursing practice often involves caring for patients with signs and symptoms that may or may not be specifically related to a medical problem. The clinical interpretation and management of these signs and symptoms are often the nurse's responsibility. As with all patients being treated for cancer, those receiving EGFR inhibitors or other targeted therapies require thorough education specific to these agents to maximize therapy. Points of emphasis should include the following:
• Therapy with these agents is intended to be chronic and may last for an extended period.
• Therapy may be combined with other novel agents or with standard chemotherapy or radiation. These combinations may result in increased expected and unexpected toxicities. Patients should be given adequate information about when to be concerned and when and how to contact the appropriate medical professional.
• Therapy may be administered as part of a clinical trial.
• Early reporting and intervention are key to managing treatment-related toxicities. Patients should be instructed how and when to report toxicities and reminded that it is easier to treat problems when they are identified early.
• Written instructions for patients and care-givers must include phone numbers of clinicians, symptoms to report, specific interventions, and what constitutes an emergency situation.
• Proper handling of oral drugs includes appropriate storage and keeping them out of the reach of children or pets.
• Patients need comprehensive lists of medications that should not be taken in conjunction with targeted therapies.
• Instructions for patients should be taken from the manufacturer's drug information but may be simplified to ensure the patient's or caregiver's complete understanding.
• Adequate dosing of targeted therapies is just as important as with cytotoxic therapy in controlling the cancer. Compliance with recommended dosing regimens is imperative for good clinical outcomes.
• Nurses should always utilize the most current dosing and toxicity information available. This information is readily available through NCI Fact Sheets, Hospital Formulary sources and individual manufacturers prescribing information provided in the drug's package insert.
Unlike many types of cancer therapies that are given for a prescribed period of time, these novel agents may be continued for an extended period. Administration may be stopped due to disease progression or unmanageable side effects. Patients are treated on an outpatient basis and may make fewer visits to the physician than patients on more standard anticancer therapies. Because these patients may not visit the clinician's office as frequently, routine telephone assessment becomes increasingly important. It is imperative that the nurse is aware of expected side effects and feels competent to assist patients in their management so that administration of the therapy is not interrupted or terminated. As with other forms of cancer therapy, a complete course of treatment is important in achieving the best possible therapeutic outcome. Frequent contact with the treatment team allows patients to feel supported and may improve compliance with the treatment regimen.
Some patients may believe that oral drugs are not as potent as standard chemotherapy and may not understand the importance of compliance and prompt reporting of side effects. They may "self-regulate" dosing to reduce side effects. Patients may also forget to take the medication, especially if they are not feeling well, elderly or have limited care giver support.. Treatment-specific tools such as treatment calendars and pill and toxicity diaries (see Appendix A [PDF], a sample patient toxicity record [printable version]) can help patients remain compliant with their therapy, keep track of toxicities, and feel more in control of their care. We sometimes even provide a written "contract" for patients to sign that provides complete instructions for drug usage and a synopsis of the patient's responsibilities surrounding medication compliance. We encourage compliance by requiring patient to return unused drug and filled out pill diaries for assessment of compliance prior to receiving a prescription for additional medication. This has proved to be an effective strategy for improving compliance with a prescribed regimen.
One strategy to assist patients in managing toxicities is to prepare a treatment-specific template of questions for medical staff to ask the patient during clinic visits or phone calls. Providing access to regularly answered e-mail is attractive to some patients for communication with clinicians and nurses. Making the current National Cancer Institute's Common Toxicity Criteria for Adverse Events available to all staff will encourage more consistent assessment of patients' toxicities. The treatment team should adopt a uniform plan for managing toxicities to reduce patients' confusion. This plan should be presented to patients and caregivers in writing.
Conclusions
The advent of new targeted drugs for the treatment of cancer is an exciting development. Further development of these agents hold the promise of truly "individualized" cancer treatment. Oral dosing of many of the drugs allows patients more freedom from the treatment center or hospital and a greater sense of participation with clinicians. Less toxic drugs reduce the debilitation associated with cytotoxic agents and can result in improved quality of life. Effective education results in improved compliance with treatment regimens and potentially improved clinical outcomes. Nursing intervention remains a vital component in the successful use of these novel agents.
Disclosures:
Jeanne Riddle has participated in nurse advisory boards for AstraZeneca and has been a speaker for the Inside Cancer series sponsored by a grant from AstraZeneca. Kathleen Gillaspy has served on advisory boards and acted as a speaker for Genentech.
References:
1. Iressa (gefitinib) prescribing information. AstraZeneca Pharmaceuticals, Wilmington, Delaware, 2005.
2. Nexavar (sorafenib) prescribing information. Bayer Pharmaceuticals, West Haven, Connecticut, 2006.
3. Nelson MH, Dolder CR: Lapatinib: A novel dual tyrosine kinase inhibitor with activity in solid tumors. Ann Pharmacother 40:261-269, 2006.
4. Sutent (Sunitinib Malate) for Health Care Professionals. Pfizer Oncology, 2006.
5. US Food and Drug Administration (January 28, 2006): FDA approves new treatment for gastrointestinal and kidney cancer (FDA News NEW01302). Washington, DC, US Government Printing Office, 2006.
6. Erbitux (cetuximab) prescribing information. ImClone Systems and Bristol-Myers Squibb Company. Branchberg and Princeton, New Jersey, 2006.
7. Vectibix (panitumumab) prescribing information. Amgen, Thousand Oaks, California, 2006.
8. National Cancer Institute Fact Sheet: Herceptin (Trastuzumab): Questions and Answers. NCI Publication 7.45. Issue Date: 06/13/2006.
9. Avastin (bevacizumab) prescribing information. Genentech, South San Francisco, 2006.
10. Baselga J: The EGFR as a target for anticancer therapy-Focus on cetuximab. Eur J Cancer 37 (supp 4):S16-S22, 2001.
11. Snyder LC, Astsaturov I, Weiner LM: Overview of monoclonal antibodies and small molecules targeting the epidermal growth factor receptor pathway in colorectal cancer. Clin Colorectal Cancer 5(suppl 2):S71-S80, 2005.
13. Morabito A, De Maio E, Di Maio M, et al: Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: Current status and future directions. Oncologist 11:753-764, 2006.
14. Hidalgo M, Siu L, Nemunaitis J, et al: Phase I and pharmacologic study of OSI-774. An epidermal growth factor receptor tyrosine kinase inhibitor in patients with advanced solid malignancies. J Clin Oncol 19:3267-3279, 2001.
15. Baselga J, Rischin D, Ranson M, et al: Phase I safety, pharmocodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solids tumor types. J Clin Oncol 20:4292-4302, 2002.
16. Common Terminology Criteria for Adverse Events (CTCAE). US Department of Health and Human Services, National Institutes of Health, National Cancer Institute.
17. Riddle J, Lee P, Purdom M: The epidermal growth factor receptor as a novel target for cancer therapy: Case studies and clinical implications. Semin Oncol Nurs 18 (4 suppl 4):11-19, 2002.
18. Saltz LB: Understanding chemotherapy-induced diarrhea. J Support Oncol 1:35-46, 2003.
19. Waxman ES: Nursing Management: Targeted Therapies, in Current Topics in Lung Cancer, pp 36-48. Miami, Institute for Medical Education and Research, 2004.
20. Miller KD, Trigo JM, Wheeler CB, et al: A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer. Clin Cancer Res 11:3369-3376, 2005.
21. Hatley TW, Platt-Mills TAE, et al: Diagnosis and management of anaphylaxis. Am J Hematol Oncol 5(7 suppl 7), 2006.
22. Tarceva (erlotinib) prescribing information. Genentech, South San Franscisco, and OSI Pharmaceuticals, Melville, New York, 2005.
12. Heymach JV, Nilsson M, Blumenschein G, et al: Epidermal growth factor receptor inhibitors in development for the treatment of non-small cell lung cancer. Clin Cancer Res 12:4441-4445, 2006.
Oncology Peer Review On-The-Go: Cancer-Related Fatigue Outcome Measures in Integrative Oncology
September 20th 2022Authors Dori Beeler, PhD; Shelley Wang, MD, MPH; and Viraj A. Master, MD, PhD, spoke with CancerNetwork® about a review article on cancer-related fatigue published in the journal ONCOLOGY®.