Toxicity of Targeted Therapy
Epidermal growth factor receptor inhibitors and other protein kinase inhibitors are novel agents that have recently been incorporated into the treatment of many solid malignancies. They specifically target the aberrant proteins in cancer cells and thus have fewer associated toxicities. These agents represent a welcome change in cancer treatment, as standard chemotherapy regimens entail numerous toxicities that may disqualify patients from continued administration or cause dose reductions or early discontinuation because of poor tolerance. Among the most common toxicities related to epidermal growth factor receptor inhibitors and multitargeted agents are cutaneous reactions that can lead to noncompliance and affect patients' sense of well-being. Oncology nurses play a key role in providing patient education, instituting preventive measures, and assuring early detection and intervention for patients on targeted therapies.
Nursing management of patients with advanced malignancies presents a formidable challenge. In addition to the discomfort and debilitation these diseases can cause, side effects of traditional treatment modalities such as surgery, chemotherapy, and radiation may lead to severe and sometimes fatal sequelae. New targeted therapies promise an effective treatment with more easily tolerated and managed side effects. Basic understanding of the drugs' mechanism of action contributes to the successful management of the toxicities that can be manifested. Effective patient education results in improved compliance with treatment regimens and potentially improved clinical outcomes. Nursing intervention remains a vital component in the successful use of these novel agents.
Oncology nurses who support patients undergoing standard chemotherapy have long hoped for a less disruptive therapy. Epidermal growth factor receptor inhibitors (EGFRIs) and multitargeted kinase inhibitors (MTKIs), collectively known as targeted therapies, compose a new class of anticancer therapies that are changing the way patients live with and fight cancer. (See the article by Carney et al in this supplement for a comprehensive list of these agents.) Oncology nurses are experiencing first-hand the difference these agents make in patient survival and improved quality of life. However, many nurses struggle with the drugs' dermatologic side effects, which are sometimes inadequately managed because of a lack of relevant data.
Failure to treat these side effects diminishes patients' quality of life and decreases their compliance, potentially leading to treatment failure. Although no data are available from controlled trials investigating the management of associated dermatologic toxicities, many oncologists and dermatologists are working toward better strategies. In the meantime, abundant literature from experts who deal with these agents and their side effects provide oncology nurses with the tools they need to support their patients.
Epidermal Growth Factor Receptor Inhibitors: Background
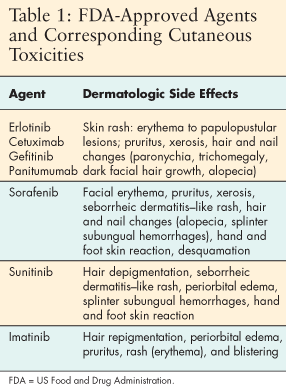
Overexpression of the epidermal growth factor receptor (EGFR) is found in many tumors and is associated with chemotherapy and radiation resistance, increased metastasis, and a poor prognosis.[1,2] The epidermal growth factor receptor is the molecular target for drugs such as cetuximab (Erbitux), panitumumab (Vectibix), and erlotinib (Tarceva), whose inhibition of EGFR has proved to be an effective anticancer therapy. Epidermal growth factor receptor inhibitors are a class of agents used to fight colorectal, pancreatic, lung, head and neck, breast, bladder, and ovarian cancers.
The US Food and Drug Administration (FDA) has approved cetuximab,[1,2] a monoclonal antibody, for the treatment of colorectal and head and neck cancer; panitumumab, which is indicated for colorectal cancer[3]; and erlotinib, a tyrosine kinase inhibitor, for pancreatic and non-small-cell lung cancer.[4] The FDA has also approved imatinib (Gleevec), sorafenib (Nexavar), and sunitinib (Sutent), agents that block several pathways.[1] Investigations of the EGFR inhibitors for use in other malignancies are under way, and numerous clinical trials are studying agents that have not yet received FDA approval.[3-7]
Dermatologic side effects are among the major toxicities associated with EGFR-targeted agents (Table 2).[8] Cutaneous reactions include papulopustular skin rash, nail and hair changes, xerosis, pruritus, and aphthous ulcers in the mouth and nasal mucosa.[8,9] The difficulty of managing these conditions is exacerbated by the fact that no randomized controlled trials studying dermatologic side effects have been conducted.
Papulopustular Eruption
The most common cutaneous reaction to EGFR inhibitors is a papulopustular skin rash, also referred to as acneiform eruption,[2] rash,[10] acne folliculitis,[11] acneiform follicular rash,[12] acnelike rash,[13] maculopapular skin rash,[14] and monomorphic pustular lesions.[15] Because the pathophysiology appears to be different from that of other documented dermatologic conditions, the term PRIDE (Papulopustules and/or Paronychia, Regulatory abnormalities of hair growth, Itching, and Dryness due to EGFR inhibitors) syndrome may be used to encompass all elements of cutaneous toxicities.[16]
While the extent of the papulopustular eruption is usually described as mild, moderate, or severe, the National Cancer Institute Common Toxicity Criteria, versions 2.0 and 3.0, using grades 1 to 4, have been used in clinical trials.[2,17,18] Approximately 50% of patients experience papulopustular eruption at grades 1/2, and up to 18% experience a grade 3/4 eruption.[1] The true incidence is uncertain because papulopustular eruption varies among trials and agents, and various terminologies are employed to describe the same phenomenon. Several studies have linked rash and degree of rash to disease response, an interesting correlation that is currently under investigation.[18,19]
The rash is characterized by pustular or papular follicular eruption. It appears most frequently on the face, neck, chest, and upper back, areas that are exposed to the sun and are rich in sebaceous glands (Figure 1).[1,2,8,9,20] Ulcers may also be noted in the mouth and nares after several weeks on therapy and can be chronic. Pruritic and inflamed lesions may develop on the scalp as well. Investigators have suggested that the rash is not associated with comedones (whiteheads or blackheads) or bacterial infection, although secondary bacterial infection sometimes occurs.[21,22]
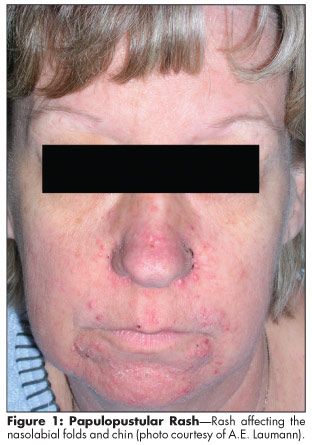
The rash usually appears within 7 to 10 days of initiation of an EGFR inhibitor. The intensity often waxes and wanes throughout therapy, but it often peaks at weeks 2 to 3.[22] The rash is most often mild to moderate (grade 1/2) with macular papular follicular eruption with or without pruritus and erythema. It may resemble rosacea with associated telangiectasia.[2] Infrequently, grade 3 or 4 rash develops, with necrosis and ulceration.
The MTKIs sunitinib and sorafenib can also cause acral erythema and edema.[1] Acral erythema and hyperkeratosis differ from those conditions as seen in hand-foot syndrome, which is associated with certain chemotherapies.[1] Acral erythema can be painful and may cause paresthesias. Periocular edema is a common side effect of imatinib and, less commonly, of sunitinib.[1]
Xerosis/Pruritus/Secondary Bacterial Infection
Xerosis, pruritus, and secondary bacterial infection may occur independently of or concurrently with the papulopustular eruption. Xerosis, or dry skin, is generalized and usually appears a few months after initiation of a targeted agent. It is often chronic throughout treatment. Secondary infection has been documented and may result from irritation of pustular lesions and xerosis, which causes thinning of the epidermis.[9] Secondary infection can also occur if pruritus exists and the patient scratches until the skin breaks.
Hair and Nail Changes
The EGFR inhibitors and MTKIs may cause hair changes, which typically appear after at least 2 to 3 months of therapy. With both types of agents, hair can become brittle and curly and grow more slowly, leading to alopecia.[1,2,8,9] With EGFR inhibitors, eyelashes may become thick and wiry (trichomegaly).[1] Patients treated with EGFR inhibitors may also experience increased growth of facial hair.
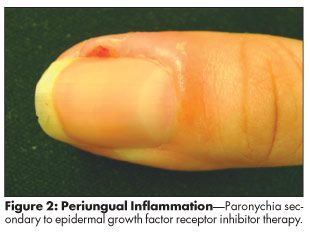
Changes in nails occur later in the treatment course, often 4 weeks to several months after initiation of EFGR inhibitor therapy.[1,2,8,9] The most common nail change is paronychia, an often painful inflammation of the nail fold that looks like an ingrown nail (Figure 2). It can affect any of the fingers or toes but is most often seen in the thumb or great toe.[1,2,8,9] Pyogenic granuloma of the nail may develop, with associated fissures and secondary bacterial infections. Reports of cracking, brittle, slow-growing nails are common with long-term therapy.[1,2,8,9] Sunitinib and sorafenib may cause subungual splinter hemorrhage, a painless black-and-red-lined appearance under the nails.[1]
Treatment Options
Nurses may encounter problems in patient compliance with these targeted agents because of their cutaneous toxicities. The orally administered agents (erlotinib, sorafenib, and sunitinib) are particularly prone to compliance issues. If patients experience uncontrolled pruritus or extreme xerosis, for example, they may resist therapy. Early identification and treatment of these side effects are therefore critical to ensure patient compliance and possibly to prevent dose reduction or discontinuation of the medication.[26]
Treatment recommendations for rash vary, depending on its grade and characteristics. Mild reactions (grade 1) may benefit from topical agents, while moderate to severe reactions (grade 2 and 3) usually require oral agents. Grade 4 rash necessitates immediate discontinuation of the EGFR inhibitor.[1,2,8,9] If toxicities of any grade affect a patient's quality of life, and appropriate management fails to alleviate symptoms, more aggressive intervention or dose reduction should be considered.
Topical steroids such as fluticasone propionate 0.5% have been used, but they should be applied sparingly and only for a short course of 2 to 4 weeks for acute exacerbation relief. For longer courses, alclometasone dipropionate cream 0.05% or hydrocortisone 2.5% ointment may be used. Because patients may be on EGFR inhibitor therapy for months to years, topical steroids should not be considered as maintenance medications, due to their long-term side effects. Regenecare is a prescription topical gel, containing collagen, aloe, vitamin E, and lidocaine, which soothes inflamed rash and provides symptomatic relief from itching, burning, and tenderness.
The papulopustular rash has not been shown to have a bacterial origin, and it responds to some oral antibiotics because of their anti-inflammatory properties.[23] Doxycycline or minocycline, 100 mg once or twice daily for 3 to 6 weeks, is the initial treatment. It may extend into a maintenance therapy of 100 mg by mouth once a day if reexacerbation occurs after discontinuation.[2,3,9,10,23]
Secondary bacterial and concurrent viral infections may arise.[1,2,8,9] Obtain cultures if vesicular rash, yellow crusting, or drainage of the rash occur. Isotretinoin, an agent used to treat acne vulgaris, has been used for the papulopustular eruption caused by EGFR inhibitors, but its high potential to induce further xerosis and paronychia has limited its usefulness.[24]
Several other medications have been tried for this application, including topical immunomodulators such as tacrolimus ointment and pimecrolimus cream. These drugs' anti-inflammatory properties may reduce erythema and help resolve pustules,[8] although patients often experience a burning sensation for the first few days. In January 2005, the FDA mandated that these agents bear a "black box" warning indicating possible cancer risks. As a result, they are recommended only after other prescription topical medicines have failed. Olux foam or Capex shampoo can be used for pruritus and inflamed lesions on the scalp, and Bacitracin nasal ointment can be applied to ulcers in the nares.
Control of itching is important because secondary infection may occur if pruritus persists and patients erode the skin by scratching. Topical and oral agents often alleviate symptoms of pruritus. Sarna Ultra Anti-Itch Cream is used for treatment of pruritus, as are oral agents such as hydroxyzine and diphenhydramine.
Opinions differ on the efficacy of topical steroids in treating paronychia.[1,2,8,9] Partial and total nail avulsion has been found to relieve inflammation, pressure, and pain,[1,2,9] but full nail extraction should be a last resort, as the nail might not regrow. Silver nitrate and cushioning have been used with success in pyogenic granuloma.[1,8,9] Band-Aid Liquid Bandage and Cordran (flurandrenolide) tape have successfully treated fissures.
Nursing Implications
Through patient education, nurses play a critical role in early intervention for cutaneous side effects of EGFR and MTK inhibitor therapies.[25] Patient education should include teaching points that help prevent or alleviate exacerbation. The website of People Living With Cancer (www.plwc.org) includes practical information on the side effects of targeted therapies.
Nurses should counsel patients about the importance of early intervention and encourage them to call if they develop any dermatologic toxicity.[26] Recommend that they avoid sun exposure and use a broad-spectrum sunscreen with an SPF ≥ 30 (eg, titanium dioxide or zinc oxide) at all times.[2]
To prevent xerosis, encourage patients to apply emollients at least twice a day after beginning therapy with an EGFR inhibitor. Creams and ointments such as Vanicream and Aquaphor are preferable to lotions. Vanicream (www.vanicream.com) is an over-the-counter product line that includes sunscreen, lotion, bath oil, shampoo, conditioner, and soap, all of which are fragrance- and chemical-free. Patients may have to ask their pharmacy to order these products, but they are available without a prescription. In cases of severe rash, wet compresses can help reduce inflammation.
To prevent paronychia, teach patients to avoid nail biting, pushing back cuticles, tearing the skin around the nail bed, and wearing tight shoes.[1,8,9] Topical antibiotics and antiseptics, combined with regular foot soaks, may prevent secondary infection.[1,8,9]
If a patient develops trichomegaly, it is helpful to trim the eyelashes. Referral to an ophthalmologist may be necessary, as corneal abrasion may occur.
Researchers are conducting studies of laser therapy for facial hair removal. If it proves to be safe and efficacious, dermatologists providing this treatment should use a lower than standard dose because of uncertain side effects of EGFR inhibitors.
Finally, always stress that patients should call as soon as they develop symptoms so that early intervention can be initiated.
Conclusion
Oncology nurses, the main source of patient education, must be aware of the toxicities associated with EGFR inhibitors and MTKIs in order to fully educate patients about them.[27] With thorough patient preparation, implementation of preventative measures, early intervention, and an interdisciplinary approach, oncology nurses can help ensure improved quality of life and compliance for patients receiving EGFR and MTK inhibitors.
Disclosures:
Mario Lacouture has acted as a consultant for ImClone Systems, and served on advisory boards for Genentech and Amgen.
References:
1. Robert C, Soria JC, Spatz, A, et al: Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 6(7):491-500, 2005.
2. Segaert S, Tabernero J, Chosidow O, et al: The management of skin reactions in cancer patients receiving epidermal growth factor receptor targeted therapies. J Dtsch Dermatol Ges 3(8):599-605, 2005.
3. Weidmann MW, Caca K: Molecularly targeted therapy for gastrointestinal cancer. Curr Cancer Drug Targets 5:171-193, 2005.
4. Johnson JR, Cohen M, Rajeshwari S, et al: Approval summary for erlotinib for treatments of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res 11:6414-6421, 2005.
5. Harrari PM: Anti-EGFR therapy update: Clinical experience and adverse events insights. Oncology 20(5 suppl 2):3-4, 2006.
6. Caponigro F, Milano A, Basile M, et al: Recent advances in head and neck cancer therapy: The role of new cytotoxic and molecular-targeted agents. Curr Opin Oncol 18(3):247-252, 2006.
7. Vokes E, Chu E: Anti-EGFR therapies: Clinical experience in colorectal, lung and head and neck cancers. Oncology 20(5):15-25, 2006.
8. Fox L: Pathology and management of dermatologic toxicities associated with anti-EGFR therapy. Oncology 20(4 suppl 2):26-34, 2006.
9. Segaert S, Van Cutsem E: Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol 16(9):1425-1433, 2005.
10. Haas-Kogan DA, Prados MD, Tihan T, et al: Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst 97(12):880-887, 2005.
11. Robert C, Soria JC, Spatz A, et al: Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 6(7):491-500, 2005.
12. Robert F, Ezekiel MP, Spencer SA, et al: Phase I study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy inpatients with advanced head and neck cancer. J Clin Oncol 19(13):3234-3243, 2001.
13. Kris MG, Natale RS, Herbst TJ, et al: Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA 290:2149-2158, 2003.
14. DeBono JS, Schwartz G, Monroe P, et al: Phase I and pharmacokinetic study of oral GW572016, a potent reversible dual inhibitor of both erbB1 and erbB2 tyrosine kinase, administered in combination with capecitabine (abstract 901). Proc Am Soc Clin Oncol 22:225, 2003.
15. Hidalgo M, Siu LL, Nemunaitis J, et al: Phase I and pharmacokinetic study of oral OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 19(13):3267-3279, 2001.
16. Lacouture ME, Lai SE: The PRIDE (Papulopustules and/or Paronychia, Regulatory abnormalities of hair growth, Itching, Dryness due to EGFR inhibitors) syndrome. Br J Dermatol 155(4):852-854, 2006.
17. National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0. Available at http://www.fda.gov/cder/cancer/toxicityframe.htm Accessed November 2, 2006.
18. Perez- Soler R, Chachoua A, Hammond L, et al: Determinants of tumor response and survival with erlotinib in patients with non-small cell lung cancer. J Clin Oncol 22(16):3238-3247, 2004.
19. Perez-Soler R, Saltz L: Cutaneous adverse effects with HER1/EGFR-targeted agents: Is there a silver lining? J Clin Oncol 23(22):5235-5246, 2005.
20. Busam KJ, Capodieci P, Motzer R, et al: Cutaneous side-effects in cancer patients treated with antiepidermal growth factor receptor antibody C225. Br J Dermatol 144:1169-1176, 2001.
21. Dick S, Crawford G: Managing cutaneous side effects of epidermal growth factor receptor (HER1/EGFR) inhibitors. Community Oncology 2:492-496, 2005.
22. Perez-Soler R, Delord JP, Halpern A, et al: HER1/EGFR inhibitor-associated rash: Future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash forum. Oncologist 10(5):345-356, 2005.
23. Suh KY, Kindler HL, Medenica M, et al: Doxycycline for the treatment of paronychia induced by the epidermal growth factor receptor inhibitor cetuximab. Br J Dermatol 154:191-192, 2006.
24. Gutzmer R, Werfel T, Mao R, et al: Successful treatment with oral isotretinoin of acneiform skin lesions associated with cetuximab therapy. Br J Dermatol 153:849-851, 2005.
25. Riddle J, Lee P, Purdom M: The epidermal growth factor receptor as a novel target for cancer therapy: Case studies and clinical implications. Semin Oncol Nurs 18(suppl 4):11-19, 2006.
26. Lacouture ME, Basti S, Patel J, et al: The SERIES Clinic: An interdisciplinary approach to the management of toxicities of EGFR inhibitors. J Support Oncol 4:236-238, 2006.
27. Lacouture ME: Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 6:803-812, 2006.
Oncology Peer Review On-The-Go: Cancer-Related Fatigue Outcome Measures in Integrative Oncology
September 20th 2022Authors Dori Beeler, PhD; Shelley Wang, MD, MPH; and Viraj A. Master, MD, PhD, spoke with CancerNetwork® about a review article on cancer-related fatigue published in the journal ONCOLOGY®.