Prevention of Colorectal Cancer: An Emerging Role for High-Dose Vitamin D Supplementation?
Colorectal cancer is the second most common cause of cancer death in the United States. It is estimated that about 55,000 patients will die this year due to advanced colorectal cancer. These grim statistics persist despite a marked increase in the rate of screening colonoscopies and improvements in adjuvant chemotherapy. Successful chemoprevention strategies may reduce the risk of new colorectal cancers, thus decreasing related overall morbidity and mortality.
Colorectal cancer is the second most common cause of cancer death in the United States. It is estimated that about 55,000 patients will die this year due to advanced colorectal cancer. These grim statistics persist despite a marked increase in the rate of screening colonoscopies and improvements in adjuvant chemotherapy. Successful chemoprevention strategies may reduce the risk of new colorectal cancers, thus decreasing related overall morbidity and mortality.
Attempts at Chemoprevention
Unfortunately, the investigation of several chemoprevention agents in polyp prevention, an imperfect surrogate of colorectal cancer risk, did not result in any major breakthroughs. A controlled randomized wheat bran supplementation trial failed to show any significant decrease in the rate of adenomatous polyp recurrence in patients with previously resected adenomas.[1] Another controlled randomized study of calcium carbonate, 3,000 mg/d, showed a moderate decrease in risk of polyp recurrence that barely reached statistical significance.[2]
Daily aspirin supplementation was also investigated in the prevention of polyp recurrence in two large controlled studies.[3,4] One randomized study showed a significant reduction in polyp recurrence among patients with resected colon cancer receiving 325 mg of aspirin per day.[4] However, the second study showed a significant reduction in polyp recurrence in a population of patients with a history of resected polyps only in the arm receiving low-dose aspirin at 81 mg/d.[3] Given the risks of bleeding associated with aspirin use, it would be unjustified to implement widespread use of low-dose aspirin for the purpose of colorectal cancer prevention.
Celecoxib (Celebrex), a selective cyclooxygenase (COX)-2 nonsteroidal inhibitor associated with a decreased risk of gastrointestinal bleeding, has been shown to reduce the risk of polyp recurrence.[5,6] However, this agent is unlikely to be used any further in large-scale prevention efforts, given its association with an increased incidence of cardiovascular events.[6]
Recently, the Women's Health Initiative (WHI) study reported on the value of vitamin D at 400 IU/d in combination with 1,000 mg/d of elemental calcium vs a matched placebo in more that 36,000 postmenopausal women.[7] Participants in this study received vitamin D or placebo for an average of 7 years. No reduction in the risk of colorectal cancer was noted on the treatment arm.[7] This is the only adequately powered prevention study so far to use colorectal cancer incidence as an outcome measure for colorectal cancer prevention. Despite the negative results from the WHI study, a growing body of preclinical and clinical evidence supports the use of vitamin D3 supplementation for the purpose of colorectal cancer prevention.
Underlying Pathophysiology
Normal, precancerous, and cancerous colonic epithelium may be targets of vitamin D through its direct effect on vitamin D receptors (VDR).[8-12] VDR expression increases in the progression from normal mucosa to premalignant or early malignant tissue (aberrant crypt foci, polyps, and differentiated adenocarcinoma).[10,12] VDR is the target of the active vitamin D compound 1,25 hydroxy-vitamin D (1,25-OH D3), and the binding of this compound to VDR results in antiproliferating and differentiating activities. The importance of VDR activation in preventing carcinogenesis has been demonstrated in several preclinical models. An inverse relationship between cellular proliferation and VDR expression was demonstrated in mouse colon.[13] Furthermore, complete loss of VDR (knockout mice) was associated with an increased proliferation and increased oxidative DNA stress, which may promote carcinogenesis.[13,14] In addition, vitamin D3 antitumor activity was documented in an APCmin mouse model.[15]
Vitamin D3 antiproliferative effects on colonic epithelium have also been demonstrated in human subjects. The supplementation of vitamin D3 in combination with calcium carbonate was shown to reduce the proliferative index and increase VDR expression in colonic polyps and mucosa.[16] Furthermore, an inverse relationship between serum 25-OH D3 levels and whole-crypt proliferative index had been previously documented.[17]
Clinical Evidence
Initial supportive clinical evidence came from a study of the geographic incidence of colorectal cancer in men in 1959-1961.[18] States with the highest solar exposure (and, hence, highest D3 synthesis) had the lowest incidence of colorectal cancer, whereas the opposite findings were true in states with the lowest sunlight exposure.[18] The association between sunlight exposure and age-specific rate of colorectal cancer was found to hold true on subsequent analysis using data from the Centers for Disease Control/National Center for Health Statistics.[19]
Sunlight is responsible for the synthesis of more than 90% of an individual's vitamin D requirement. During sun exposure, ultraviolet B is absorbed by 7-dehydrocholesterol in the skin, resulting in its conversion to previtamin D3, which is rapidly isomerized by the body temperature to vitamin D3. Vitamin D3 is in turn converted in the liver to 25-OH D3, which is converted in the kidneys and at the local tissue level into the active compound 1,25-OH D3. The less sun exposure, the higher the risk of vitamin D3 depletion. It was thus hypothesized that decreased sunlight exposure increases the risk of colorectal cancer indirectly by inducing a state of vitamin D deficiency.
Several case-control studies have evaluated the significance of vitamin D status and risk of colorectal cancer. Four studies evaluated 25-OH D3 levels (a reliable assay of overall vitamin D status) in colorectal cancer patients and compared them to matched controls.[20-23] In all four studies, a trend or a definite association existed between lower 25-OH D3 levels and colorectal cancer. A meta-analysis of the four case control studies suggests that concentrations of 25-OH D3 exceeding 33 ng/mL (82 nm/L) would reduce the risk of colorectal cancer by 50%.[24]
An analysis of 14 studies assessing vitamin D intake and risk of colorectal cancer similarly showed that higher supplementation is associated with protection from colorectal cancer. The median dose among these 14 studies that resulted in 50% reduction in colorectal cancer was estimated at 1,000 IU/d.[24] These findings are supported further by the nested case control study within WHI, where a strong inverse association was noted between 25-OH D3 levels and the incidence of colorectal cancer.[7] The highest quartile (25-OH D3 ≥ 58.4 nmol/L) in this group had the lowest risk for colorectal cancer.[7]
Limitations of the WHI Study
Putting things in perspective, we believe that the WHI study was likely to fail in preventing colorectal cancer. The 400-IU/d dose of vitamin D3 that was investigated by the WHI was inadequate based on epidemiologic studies. It has been estimated that 40 IU/d of vitamin D3 will increase plasma 25-OH D3 levels by 0.7 nmol/L above steady state.[25] Thus, a 400-IU/d dose will increase the steady state of 25-OH D3 by 7 nmol.
The population in the WHI study had an estimated median 25-OH D3 level of 42 nmol/L (by extrapolation from the nested case-control study). The 400 IU/d of vitamin D3 supplementation would have increased the median to 49 nmol/L-well below the target of 82 nmol/L associated with a 50% reduction in colorectal cancer incidence.[24] To ensure that more than 75% of the nested case-control population in the WHI achieves 25-OH D3 levels exceeding 82 nmol/L, the upper limit of the lowest quartile (31 nmol/L) would have to be raised by 50 nmol/L; this translates to 1,400 IU of vitamin D3 supplementation.
Of note, the recommended daily allowance of vitamin D in the United States was previously set at 200 IU/d, but the official safety limit has been set at 2,000 IU/d. Many have argued, however, that significantly higher doses can be administered safely to the general population.[25,26]
RPCI Study
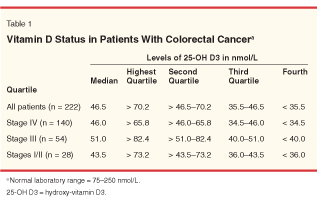
We recently evaluated the 25-OH D3 status in 222 patients treated in the colorectal cancer clinic at Roswell Park Cancer Institute. Serum samples were immunoassayed in a commercially available standardized test (normal recommended range: 75-250 nmol). Extreme vitamin D deficiencies were noted in the overall population, with the median 25-OH D3 level being 46.5 nmol/L. Deficiencies were seen across the board for all stages of disease (Table 1). Only the upper quartile (25-OH D3 > 70 nmol/L) of the tested population approached the normal recommended range of 75 nmol/L for optimal vitamin D status. Our results confirm the severe vitamin D deficiency status of patients with colorectal cancer and thus the need for higher doses of vitamin D3.
Vitamin D in Advanced Disease
While significant emphasis has been placed on a potential role for vitamin D in the prevention of colorectal cancer, an equally important role for vitamin D replacement may exist in patients with locally advanced or metastatic disease. Vitamin D-replete status has been associated with a variety of health benefits including a reduced risk of cardiovascular death, amelioration of hypertension, reduction in insulin resistance, and a decreased risk of osetoporotic fractures.[27,29] A meta-analysis of randomized studies in vitamin D supplementation showed a decreased risk of fractures only with higher vitamin D doses and in association with 25-OH D3 levels exceeding 75 nmol/L.[30] Cardiovascular events, insulin resistance, and osteoporosis are seen increasingly with prolonged use of chemotherapy and accompanying antiemetic steroid use in patients with advanced colorectal cancer.
With a steadily improving median overall survival credited to the addition of novel chemotherapeutic agents, vitamin D supplementation is an easy intervention that may reduce the risks of these comorbidities. Vitamin D status may also have a direct impact on the overall survival of patients with cancer. In a 456-patient cohort undergoing surgery for early lung cancer in the Boston area, those who were operated upon during the summer season and who were on a diet rich in vitamin D had a better survival compared with the rest of the population.[31]
Furthermore, a prospective study of docetaxel (Taxotere) with or without pulse calcitriol (1,25-OH D3) in patients with refractory prostate cancer has shown a survival advantage in the calcitriol arm as well as a reduction in grade 3/4 toxicities.[32] It is unknown whether a replete vitamin D status would reduce the rate of cancer growth or enhance the effect of chemotherapy. It has been recently shown that the growth of a colon cancer xenograft was faster in mice fed a vitamin D-depleted diet vs vitamin D-replete mice.[33]
Recommendations and Conclusions
We currently recommend determining 25-OH D3 status of all colorectal cancer patients. Our practice has been to give vitamin D-deficient patients 2,000 IU/d of vitamin D3 and to recheck their levels in 3 months, when a steady-state level would be expected. In patients who do not achieve a target level of 75 nmol/L, we recommend the escalation of vitamin D3 up to doses of 4,000 IU/d with serial 25-OH D3 levels to determine response.
The role of vitamin D in colorectal cancer prevention, toxicity amelioration, and improvement in outcome is yet to be determined conclusively. Future prevention studies should investigate doses of vitamin D3 that exceed 2,000 IU/d to adequately assess the hypothesis of vitamin D in prevention. These studies should also take into account other variables that affect an individual's vitamin D status such as age, sun exposure, geographic location, season, race, and diet.
We would also hope that future prospective prevention studies will explore genomic variants in VDR (polymorphism) and vitamin D-metabolizing enzymes such as CYP24 for the purpose of identifying populations who achieve the greatest or least benefit from vitamin D supplementation.[34,35] Parallel vitamin D3 supplementation studies should be conducted in patients with advanced colorectal cancer to prospectively determine the impact of vitamin D repletion on chemotherapy toxicity, quality of life, and survival.
-Marwan Fakih, MD
-Annette Sunga, MD, MPH
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Alberts DS, Martinez ME, Roe DJ, et al: Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians' Network. N Engl J Med 342:1156-1162, 2000.
2. Baron JA, Beach M, Mandel JS, et al: Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med 340:101-107, 1999.
3. Baron JA, Cole BF, Sandler RS, et al: A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 348:891-899, 2003.
4. Sandler RS, Halabi S, Baron JA, et al: A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 348:883-890, 2003.
5. Arber N, Eagle CJ, Spicak J, et al: Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 355:885-895, 2006.
6. Bertagnolli MM, Eagle CJ, Zauber AG, et al: Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med 355:873-884, 2006.
7. Wactawski-Wende J, Kotchen JM, et al: Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 354:684-696, 2006.
8. Thomas MG, Sylvester PA, Newcomb P, et al: Vitamin D receptor expression in colorectal cancer. J Clin Pathol 52:181-183, 1999.
9. Meggouh F, Lointier P, Pezet D, et al: Evidence of 1,25-dihydroxyvitamin D3-receptors in human digestive mucosa and carcinoma tissue biopsies taken at different levels of the digestive tract, in 152 patients. J Steroid Biochem 36:143-147, 1990.
10. Cross HS, Bajna E, Bises G, et al: Vitamin D receptor and cytokeratin expression may be progression indicators in human colon cancer. Anticancer Res 16:2333-2337, 1996.
11. Evans SR, Nolla J, Hanfelt J, et al: Vitamin D receptor expression as a predictive marker of biological behavior in human colorectal cancer. Clin Cancer Res 4:1591-5, 1998.
12. Matusiak D, Murillo G, Carroll RE, Mehta RG, Benya RV. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1{alpha}-hydroxylase in normal and malignant human colon. Cancer Epidemiol Biomarkers Prev 14:2370-2376, 2005.
13. Kallay E, Bareis P, Bajna E, et al: Vitamin D receptor activity and prevention of colonic hyperproliferation and oxidative stress. Food Chem Toxicol 40:1191-1196, 2002.
14. Kallay E, Pietschmann P, Toyokuni S, et al: Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis 22:1429-1435, 2001.
15. Huerta S, Irwin RW, Heber D, et al: 1alpha,25-(OH)(2)-D(3) and its synthetic analogue decrease tumor load in the Apc(min) mouse. Cancer Res 62:741-746, 2002.
16. Holt PR, Bresalier RS, Ma CK, et al: Calcium plus vitamin D alters preneoplastic features of colorectal adenomas and rectal mucosa. Cancer 106:287-296, 2006.
17. Holt PR, Arber N, Halmos B, et al: Colonic epithelial cell proliferation decreases with increasing levels of serum 25-hydroxy vitamin D. Cancer Epidemiol Biomarkers Prev 11:113-119, 2002.
18. Garland CF, Garland FC: Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 9:227-231, 1980.
19. Harris DM, Go VL: Vitamin D and colon carcinogenesis. J Nutr 134(12 suppl):3463S-3471S, 2004.
20. Garland CF, Comstock GW, Garland FC, et al: Serum 25-hydroxyvitamin D and colon cancer: Eight-year prospective study. Lancet 2:1176-1178, 1989.
21. Braun MM, Helzlsouer KJ, Hollis BW, et al: Colon cancer and serum vitamin D metabolite levels 10-17 years prior to diagnosis. Am J Epidemiol 142:608-611, 1995.
22. Tangrea J, Helzlsouer K, Pietinen P, et al: Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control 8:615-625, 1997.
23. Feskanich D, Ma J, Fuchs CS, et al: Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 13:1502-1508, 2004.
24. Gorham ED, Garland CF, Garland FC, et al: Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol 97:179-194, 2005.
25. Heaney RP, Davies KM, Chen TC, et al: Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77:204-210, 2003.
26. Vieth R: Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 69:842-856, 1999.
27. Shoji T, Shinohara K, Kimoto E, et al: Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant 19:179-184, 2004.
28. Krause R, Buhring M, Hopfenmuller W, et al: Ultraviolet B and blood pressure. Lancet 352:709-710, 1998.
29. Chiu KC, Chu A, Go VL, et al: Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 79:820-825, 2004.
30. Bischoff-Ferrari HA, Willett WC, Wong JB, et al: Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA 293:2257-2264, 2005.
31. Zhou W, Suk R, Liu G, et al: Vitamin D is associated with improved survival in early-stage non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev 14:2303-2309, 2005.
32. Beer TM, Myrthue A: Calcitriol in the treatment of prostate cancer. Anticancer Res 26(4A):2647-2651, 2006.
33. Tangpricha V, Spina C, Yao M, et al: Vitamin D deficiency enhances the growth of MC-26 colon cancer xenografts in Balb/c mice. J Nutr 135:2350-2354, 2005.
34. Slattery ML, Murtaugh M, Caan B, et al: Associations between BMI, energy intake, energy expenditure, VDR genotype and colon and rectal cancers (United States). Cancer Causes Control 15:863-872, 2004.
35. Wjst M, Altmuller J, Faus-Kessler T, et al: Asthma families show transmission disequilibrium of gene variants in the vitamin D metabolism and signalling pathway. Respir Res 7:60, 2006.