Targeted Therapeutics Have a Role to Play in Stem Cell Transplants in Heavily Pretreated Non-Hodgkin’s Lymphoma
This special supplement to Oncology NewsInternational includes updated results ofstudies with anti-CD20 therapy and othertargeted therapies in the treatment oflymphomas, chronic lymphocytic leukemia,and immune thrombocytopenic purpura. Theresults were presented at the American Societyof Hematology 44th Annual Meeting inPhiladelphia, December 6 to 10, 2002.
DUARTE, California-Radioimmunotherapyprovides a uniqueopportunity to deliver systemic radiotherapyconcurrently with immunotherapy,offering efficacy comparablewith that of total body irradiationwhile reducing the toxicity. The ibritumomabtiuxetan (Zevalin) regimenis a radioimmunotherapeutic treatmentthat employs rituximab (Rituxan)in addition to indium-111 radiolabeledibritumomab tiuxetan(111In-ibritumomab tiuxetan), andyttrium-90 radiolabeled ibritumomabtiuxetan (90Y-ibritumomab tiuxetan).Because the therapeutic dose ofradiation is targeted to a specific celltype (ie, CD20+ B cells), it reduces apatient's risk for toxicities associatedwith total body irradiation.A phase I/II trial tested this highdoseregimen in combination withhigh-dose etoposide and cyclophosphamide(Cytoxan, Neosar), followedby autologous stem cell transplantation,in patients with poor-risk orrelapsed B-cell non-Hodgkin's lymphoma(NHL).[1] Auayporn Nademanee,MD, of City of Hope NationalMedical Center in Duarte, California,presented data showing that the additionof a high-dose ibritumomab tiuxetan-rituximab regimen to high-doseetoposide and cyclophosphamidedoes not increase transplant-relatedtoxicity and does not delay engraftment(ASH abstract 679).[1]
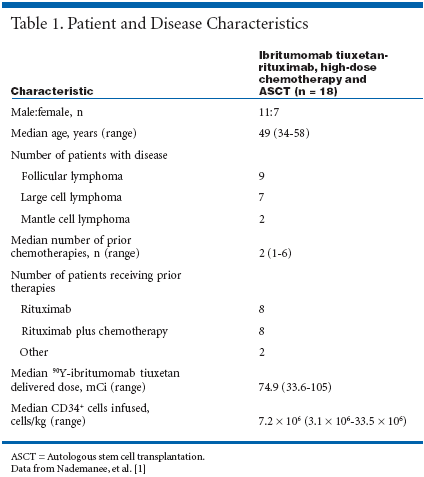
Countdown to TransplantTwenty-six patients were enrolledand 18 patients were treated. Patientand disease characteristics are summarizedin Table 1.[1]On day -21 before autologous stemcell transplantation, patients weretreated with an intravenous infusionof 250 mg/m2 rituximab to clear peripheralB cells and improve ibritumomabtiuxetan biodistribution, followedby dosimetry with 5 mCi111In-ibritumomab tiuxetan. Oneweek later, patients received 40 to 100mCi 90Y-ibritumomab tiuxetan toobtain a target dose of no greater than1,000 cGy to normal organs, combinedwith 5 mCi of 111In-ibritumomabtiuxetan.High-dose etoposide (40 to 60 mg/kg) was administered 4 days beforetransplant and high-dose cyclophosphamide(100 mg/kg), 2 days beforetransplant.Stem cells were reinfused when theradiation dose to the reinfused stemcells was estimated to be less than5 cGy. The median delivered dose of90Y-ibritumomab tiuxetan was 74.9mCi (range, 33.6 to 105 mCi).Treatment Well ToleratedThe treatment was well tolerated.Mucositis, neutropenic fever, and rashwere the most common acute toxicities.There were no transplant-relateddeaths.All patients achieved engraftment.The median time to reach an absoluteneutrophil count above 500/μL was10 days (range, 8 to 17 days) and themedian time to reach a platelet countabove 20,000/μL was 18 days (range,12 to 123 days). These median timesare similar to those reported for patientswith high-risk, persistent, orrelapsed NHL who received mobilizedperipheral blood stem cell orautologous bone marrow transplants.[2]All seven patients with active diseaseat stem cell transplantationachieved complete remission. Seventeenof 18 treated patients were aliveand in remission after a median follow-up of 8 months (range, 1 to 24months). In addition, the 1-year estimatedoverall survival and diseasefreesurvival were both 92%.This preliminary study suggeststhat this novel treatment regimen maybe effective in heavily pretreated patientswith refractory NHL who areeligible to receive stem cell transplantation.
References:
1.
Nademanee A, Molina A, FormanSJ, et al: A phase I/II trial of highdoseradioimmunotherapy (RIT) withZevalin in combination with highdoseetoposide (VP-16) and cyclophosphamide(CY) followed by autologousstem cell transplant (ASCT)in patients with poor-risk or relapsedB-cell non-Hodgkin’s lymphoma(NHL) (abstract 679). Blood 100:182a,2002.
2.
Vose JM, Sharp G, Chan WC, etal: Autologous transplantation for aggressivenon-Hodgkin’s lymphoma:results of a randomized trial evaluatinggraft source and minimal residualdisease. J Clin Oncol 20:2344-2352,2002.
Oncology Peer Review On-The-Go: Minority Treatment Disparities and Clinical Trial Enrollment
July 6th 2020The first episode of CancerNetwork's podcast Oncology Peer Review On-The-Go explores disparities in cancer care treatment among minorities and the significance of a representative sample in clinical trials.