Bcl-2 Antisense Therapy in Multiple Myeloma
Most malignant plasma cells overexpress Bcl-2, which contributesto resistance against apoptosis induced by dexamethasone and otheranticancer agents. Oblimersen sodium (Genasense, previously knownas G3139), an antisense oligonucleotide that specifically binds to bcl-2messenger RNA, decreases production of Bcl-2 protein in both humanmyeloma cell lines, as well as in ex vivo purified myeloma cells, andenhances the cytotoxicity of dexamethasone and doxorubicin. Combiningoblimersen with other anticancer agents represents a therapyenhancingstrategy to reverse the multidrug resistance seen in multiplemyeloma (MM). Phase II trials are evaluating the potential role ofoblimersen in reversing resistance to standard therapies. Preliminaryresults from these trials in patients with refractory or relapsed MMindicate that the combination of oblimersen with dexamethasone/thalidomide (Thalomid) or vincristine/doxorubicin/dexamethasone isactive and well tolerated and that oblimersen may help overcome chemotherapyresistance and restore sensitivity to MM cells. A randomizedphase III clinical trial comparing dexamethasone plus oblimersenwith dexamethasone alone in patients with relapsed or refractory myelomahas completed enrollment, with results expected to be availablein 2004. Future studies will focus on the role of oblimersen in combinationwith novel biologic agents such as bortezomib (Velcade).
ABSTRACT: Most malignant plasma cells overexpress Bcl-2, which contributesto resistance against apoptosis induced by dexamethasone and otheranticancer agents. Oblimersen sodium (Genasense, previously knownas G3139), an antisense oligonucleotide that specifically binds to bcl-2messenger RNA, decreases production of Bcl-2 protein in both humanmyeloma cell lines, as well as in ex vivo purified myeloma cells, andenhances the cytotoxicity of dexamethasone and doxorubicin. Combiningoblimersen with other anticancer agents represents a therapyenhancingstrategy to reverse the multidrug resistance seen in multiplemyeloma (MM). Phase II trials are evaluating the potential role ofoblimersen in reversing resistance to standard therapies. Preliminaryresults from these trials in patients with refractory or relapsed MMindicate that the combination of oblimersen with dexamethasone/thalidomide (Thalomid) or vincristine/doxorubicin/dexamethasone isactive and well tolerated and that oblimersen may help overcome chemotherapyresistance and restore sensitivity to MM cells. A randomizedphase III clinical trial comparing dexamethasone plus oblimersenwith dexamethasone alone in patients with relapsed or refractory myelomahas completed enrollment, with results expected to be availablein 2004. Future studies will focus on the role of oblimersen in combinationwith novel biologic agents such as bortezomib (Velcade).
Multiple myeloma (MM) remainsincurable with conventionalchemotherapy,with a median survival between 2 and3 years.[1] MM is typically chemosensitiveearlier in its clinical course butis ultimately characterized by the developmentof multidrug resistance inalmost all patients. There is growingevidence that the apoptosis-regulatingBcl-2 protein may play an importantrole in the development of multidrugresistance in patients with MM. Bcl-2antisense therapy may therefore providea new therapeutic approach toreverse this resistance and to potentiateantitumor effects of chemotherapyin the treatment of MM.[2]Bcl-2 Protein and MultidrugResistance in Multiple MyelomaBcl-2 is a conserved, ubiquitousprotein associated with the inner mitochondrialmembranes.[3] It exhibits aregulatory role in apoptosis by blockingthe release of cytochrome c.[4]Most human myeloma cell lines andsamples obtained from patients withMM overexpress Bcl-2 protein.[5,6]Various preclinical studies havedemonstrated that the Bcl-2 proteinplays an important role in mediating resistanceof MM cells to apoptosis inducedby dexamethasone and cytotoxicagents.[7-13] Tian and Gazitt[14] initiallydemonstrated that the extent ofdexamethasone-induced apoptosis inMM cell lines in vitro was inverselycorrelated with intracellular levels ofBcl-2. Subsequently, these investigatorstransfected dexamethasone-sensitive,low Bcl-2-expressing MM celllines with a Bcl-2-inducible gene constructexpressed under the control ofa lac repressor operon.[13] Activationof the inducible gene resulted in increasedintracellular levels of Bcl-2,enhanced cell growth, and decreasedspontaneous apoptosis, with concomitantincreased resistance to dexamethasone.Conversely, inactivation of theinducible gene restored sensitivity todexamethasone-induced apoptosis.Collectively, these seminal studiesdemonstrated a potential role of Bcl-2in apoptosis regulation of MM celllines as well as development of resistanceto dexamethasone-inducedapoptosis in these cells. This providesthe basis for the hypothesis that Bcl-2antisense therapy might decrease Bcl-2 protein production and thereby facilitateapoptosis in malignant myelomacells.
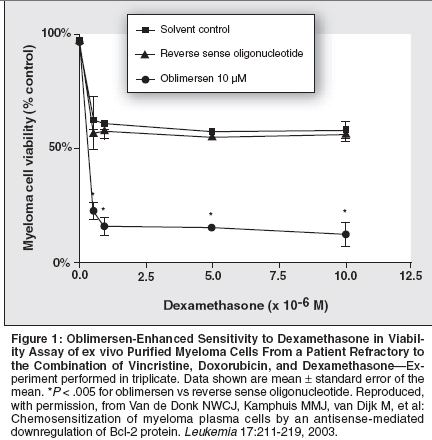
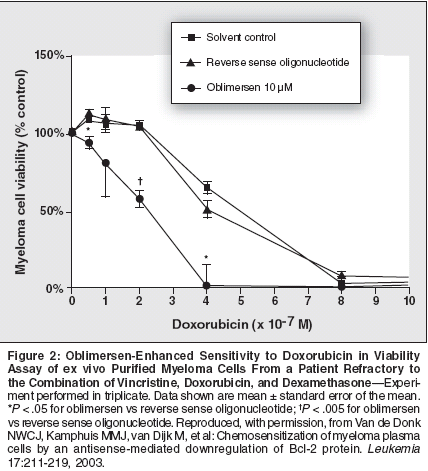
Preclinical Studies WithOblimersen SodiumOblimersen sodium (Genasense,previously known as G3139) is a Bcl-2 antisense oligonucleotide designedto specifically bind to the bcl-2 messengerRNA.[15] It binds to the firstsix codons of the human bcl-2 mRNA,forming a heterodimer. This doublestrandedmRNA is perceived as aberrantand is subsequently degraded inthe cell's cytoplasm. The result is adecreased production of the Bcl-2 proteinby the ribosome.Recent preclinical studies haveevaluated the potential of oblimersento decrease Bcl-2 protein in MM cellsand to sensitize or reverse resistanceof MM cells to agents active againstMM. Results from these studies indicatethat oblimersen is taken up by myelomacells and can decrease Bcl-2protein production. Thus, the sensitivityof myeloma cells to therapeuticagents commonly used in the treatmentof MM, such as dexamethasoneand doxorubicin, is enhanced, providinga rationale for conducting clinicaltrials using oblimersen in patients withrefractory MM.Liu and Gazitt[16] reported the effectsof pretreatment with oblimersenon dexamethasone-, paclitaxel-, and adenovirusp53-induced apoptosis andintracellular levels of Bcl-2 in myelomacells expressing varying levels of Bcl-2. Multiple myeloma cells were treatedwith oblimersen (10 μg/mL) for 3 days,followed by exposure to dexamethasone,paclitaxel, or adenovirus p53 forup to 2 additional days. In myelomacells expressing relatively low levels ofBcl-2, oblimersen exposure resulted insubstantial apoptosis with a concomitantdecrease of Bcl-2 protein. This decreasein Bcl-2 protein levels and theincreased apoptotic events were timeandconcentration-dependent, andapoptosis was noted to be mediatedthrough activation of caspase-9 andcaspase-3 and by the release of cytochromec into the cytosol.In another experiment with highBcl-2-expressing myeloma cell lines(ARH-77, U266), oblimersen pretreatmentfollowed by exposure to dexamethasoneor paclitaxel resulted in asubstantial increase in the percentage ofmyeloma cells undergoing apoptosiswhen compared with the effectsobtained with each agent with priorexposure to oblimersen. The increasedapoptosis was associated with a decreasein Bcl-2 protein. Similar resultswere obtained when freshly isolatedmyeloma cells from patients were used.In another study, van de Donk etal[17] also evaluated the effects of decreasedBcl-2 protein in ex vivo purifiedmalignant plasma cells from patientswith MM. Following incubationof the cells with oblimersen, but not withsolvent or the sense oligonucleotides, asubstantial reduction (> 75%) of bcl-2mRNA levels occurred after 2 and 4days of exposure as measured by realtimepolymerase chain reaction. Exposureof the cells to oblimersen resultedin a sequence-specific reduction ofBcl-2 protein within 4 days in 10 of11 patient samples. Significant enhancementof dexamethasone- ordoxorubicin-induced apoptosis andcytotoxicity was also noted in theseexperiments (Figures 1 and 2).O'Connor et al[18] were the firstto report on the beneficial antitumoreffects of oblimersen when combinedwith the biologic agent bortezomib(Velcade) in non-Hodgkin's lymphoma(NHL) and MM cell lines.These authors recently shared the preliminaryresults of in vitro and in vivoexperiments with oblimersen andbortezomib with established MM(U266, RPMI 8226, KMS-11) andNHL (Raji, SKI-DLCL-1, DOHH2)cell lines. These seminal experimentsshowed that oblimersen enhances theantiproliferative effect of bortezomibin MM cell lines. The augmentedantiproliferative response of oblimersenwas particularly evident in theNHL cells.In a xenograft experiment withSKI-DLCL-1 comparing oblimersenalone, bortezomib alone, and oblimersenprior to bortezomib administration,mice receiving either agentalone showed 20% to 25% inhibitionof tumor growth, whereas animals receivingthe combination experienceda reduction in tumor growth of approximately50% at 24 days. Thiseffect of oblimersen was noted to besequence-specific. Based on these experiments,future studies are proposedto explore the role of oblimersen inoptimizing the use of bortezomib inpatients with B-cell malignant disorders.Clinical Studies of OblimersenBased on the potential role of antiapoptoticBcl-2 protein in MM and theencouraging preclinical studies withoblimersen, several clinical trials in patientswith relapsed or refractory MMwere initiated. These clinical studies aredesigned to evaluate the potential roleof oblimersen to enhance sensitivityor reverse resistance to standard MMtherapies, including dexamethasone,dexamethasone/thalidomide (Thalomid),and the combination of vincristine,doxorubicin (Adriamycin), anddexamethasone (VAD).Phase II Trials
Badros et al[19,20] are conductinga phase I/II clinical trial evaluating theadministration of oblimersen followedby dexamethasone/thalidomide in patientswith relapsed or refractory MM.This study has been active as of May2004, with a target accrual of up to 46patients. To be eligible, patients mayhave received no more than four priorchemotherapy regimens.Oblimersen (7 mg/kg/d) is administeredby continuous intravenous infusionon days 1 to 7 (the first threepatients received 5 mg/kg/d), withdexamethasone (40 mg/d) given ondays 4 to 7 and thalidomide (100 to400 mg/d) starting on day 4. Treatmentcycles are repeated every 3 weeks.After three induction cycles, respondingpatients continue oblimersen on a5-week cycle with dexamethasone (20mg/d) for 4 days and thalidomide atthe tolerated dose for up to 1 year.
Preliminary data have been reportedfor the first 18 patientstreated.[20] The median age was 58years (range: 47 to 74), and 11 patientswere male. Patients had received amedian of 3 prior regimens (range:2 to 4 regimens), including prior autologous(n = 15) or allogeneic (n = 1)stem cell transplantation. Eight patientshad received thalidomide previously,for a median duration of 6.5months (range: 2 to 10 months), with6 of these patients demonstrating diseaseprogression while receiving thalidomide.Ten patients had complexkaryotype.Of the enrolled patients, 16 havecompleted the induction phase. Twopatients had a complete response, twoothers had a near-complete response,and five patients had a partial response.The overall response rate of 75% is notablein a group of patients with advanceddisease who had failed multipleprior therapies. At a median follow-upof 4 months (range: 1.5 to 8.5 months),1 responding patient had relapsed and11 continued on study therapy. Bonemarrow with adequate plasma cellswas available for 11 patients; in thisstudy no significant decrease in Bcl-2protein was observed at days 4 to 7 orday 28 of oblimersen infusion comparedwith baseline. In addition, therewere no detectable differences betweenresponders and nonresponderswith respect to Bcl-2 protein.The combination of oblimersen, dexamethasone,and thalidomide waswell-tolerated. Oblimersen toxicitiesincluded reversible increases in serumcreatinine (to > 2 mg/dL) in 10 patients,which required oblimersen dose reductionto 3 to 5 mg/kg/d, and thrombocytopeniain 6 patients. No grade 4toxicities occurred, and the majority oftoxicities were reversible.In another phase II trial, the sameconcept of restoring chemosensitivity inpatients refractory to standard chemotherapyby administration of oblimersento decrease Bcl-2 protein production incombination with chemotherapy is beingevaluated.[21] Ten patients with refractoryMM, including eight patientswhose disease was refractory to VAD,were treated with oblimersen (7 mg/kg/d) by continuous intravenous infusionfor 7 days in combination with VAD.All patients were heavily pretreated andhad received a median of 4 previouschemotherapy regimens (range: 2 to 6).Four patients had a partial response,and three patients had a minor response.Median disease progression-free survivalwas approximately 6 months(range: 2 to 7+ months), and medianoverall survival had not been reached.Oblimersen decreased the amount ofBcl-2 protein in peripheral bloodmyeloma cells, T cells, B cells, andmonocytes. The oblimersen/VAD combinationwas feasible and well tolerated.The median number of treatment cycleswas 2.5. Baseline Bcl-2 protein in bonemarrow, Ki-67 growth fraction, and thepresence of deletion of chromosome 13were not predictive of response. Thesepreliminary results suggest thatoblimersen may help to overcome chemotherapyresistance and restore sensitivityof myeloma tumor cells toVAD chemotherapy.Phase III Trial
A randomized phase III trial of dexamethasonewith or withoutoblimersen in patients with relapsedor refractory MM (GMY302) recentlycompleted accrual (n = 220). Patientscould have had up to six prior therapies.In a 4-week induction period, oraldexamethasone (40 mg) was givenevery day for 4 days of weeks 1, 2,and 3 to both cohorts. Patients whowere randomized to the study arm receivedoblimersen (7 mg/kg/d) as acontinuous infusion for 7 days onweeks 1 and 3, with dexamethasoneinitiated on day 4 of the oblimerseninfusion. Subsequent cycles in bothcohorts incorporated only one 4-daydexamethasone pulse every 3 weeks.The primary end point is time to diseaseprogression. Results of this studyare expected to be presented at theAnnual Session of the American Societyof Hematology (ASH) in 2004.ConclusionBcl-2 protein confers a clinicallyrelevant chemoresistant phenotype onmany types of cancer cells, includingmultiple myeloma, making it a relevanttarget for MM therapy. Preclinicalstudies indicate that oblimersendecreases Bcl-2 protein in MM celllines as well as in ex vivo MM cellsfrom patients and enhances the cytotoxicpotential of dexamethasone,doxorubicin, and bortezomib.Preliminary data from phase IIclinical trials in patients with refractoryor relapsed MM are encouraging.Clinically oblimersen appears to enhancethe cytotoxic potential ofantimelanoma therapies and is notedto have an acceptable safety profile inthese patients; the results of a fullyaccrued phase III trial comparingdexamethasone plus oblimersen todexamethasone alone in patients withrefractory or relapsed disease areawaited with considerable interest.Results from these ongoing studieswill determine the role of oblimersenin the treatment of patients with MM.Future studies will focus on combiningoblimersen with other novel biologicagents such as bortezomib.
Disclosures:
Dr. Chanan-Khan hasreceived grant/research support from, andconsulted for, Amgen. He is also a member ofthe speaker's bureaus of IDEC, Genentech,Celgene, and Amgen and has received otherfinancial support from Genta Incorporated.
References:
1.
Pratt G: Molecular aspects of multiplemyeloma. J Clin Pathol Mol Pathol 55:273-283, 2002.
2.
Bloem A, Lockhorst H: Bcl-2 antisensetherapy in multiple myeloma. Path Biol 47:216-220, 1999.
3.
Krajewski S, Tanaka S, Takayama S, etal: Investigation of the subcellular distributionof the bcl-2 oncoprotein: Residence in thenuclear envelope, endoplasmic reticulum, andouter mitochondrial membrane. Cancer Res53:4701-4714, 1993.
4.
Reed JC: Dysregulation of apoptosis incancer. J Clin Oncol 17:2941-2953, 1999.
5.
Ong F, van Nieuwkoop JA, de Groot-Swings GMJS, et al: Bcl-2 protein expressionis not related to short survival in multiple myeloma.Leukemia 9:1282-1284, 1995.
6.
Pettersson M, Jernberg-Wiklund H,Larsson L-G, et al: Expression of the bcl-2 genein human myeloma cell lines and normal plasmacells. Blood 79:495-502, 1992.
7.
Feinman R, Koury J, Thames M, et al:Role of NF-kB in the rescue of multiple myelomacells from glucocorticoid-inducedapoptosis by bcl-2. Blood 93:3044-3052, 1999.
8.
Gazitt Y, Rothenberg ML, Hilsenbeck SG,et al: Bcl-2 overexpression is associated withresistance to paclitaxel, but not gemcitabine, inmultiple myeloma cells. Int J Oncol 13:839-848, 1998.
9.
Hu W-X, Gazitt Y: bcl-2 plays a majorrole in resistance to dexamethasone inducedapoptosis in multiple myeloma cell lines. Int JOncol 9:375-381, 1996.
10.
Iyer R, Ding LM, Batchu RB, et al:Antisense p53 transduction leads tooverexpression of bcl-2 and dexamethasoneresistance in multiple myeloma. Leuk Res27:73-78, 2003.
11.
Panaretakis T, Pokrovskaja K, ShoshanMC, et al: Interferon-α-induced apoptosis inU266 cells is associated with activation of theproapoptotic Bcl-2 family members Bak andBax. Oncogene 22:4543-4556, 2003.
12.
Schwarze MMK, Hawley RG: Preventionof myeloma cell apoptosis by ectopic bcl-2expression or interleukin 6-mediated up-regulationof bcl-XL. Cancer Res 55:2262-2265, 1995.
13.
Tian E, Hu W-X, Gazitt Y: bcl-2 plays acritical role in growth and in spontaneous orinduced apoptosis in myeloma cell lines: Astudy with inducible bcl-2 transfection constructs.Int J Oncol 9:165-169, 1996.
14.
Tian E, Gazitt Y: The role of p53, bcl-2and bax network in dexamethasone inducedapoptosis in multiple myeloma cell lines. Int JOncol 8:719-726, 1996.
15.
Klasa RJ, Gillum AM, Klem RE, et al:Oblimersen Bcl-2 antisense: Facilitatingapoptosis in anticancer treatment. AntisenseNucleic Acid Drug Develop 12:193-213, 2002.
16.
Liu Q, Gazitt Y: Potentiation ofdexamethasone-, paclitaxel-, and Ad-p53-induced apoptosis by Bcl-2 antisenseoligodeoxynucleotides in drug-resistant multiplemyeloma cells. Blood 101:4105-4114, 2003.
17.
van de Donk NWCJ, Kamphuis MMJ,van Dijk M, et al: Chemosensitization of myelomaplasma cells by an antisense-mediateddownregulation of Bcl-2 protein. Leukemia17:211-219, 2003.
18.
O’Connor O, Srinivasan S, HernandezF, et al: Oblimersen (Bcl-2 antisense) enhancesthe antitumor activity of bortezomib (Bor) inmultiple myeloma (MM) and non-Hodgkin’slymphoma (NHL) preclinical models (abstract628). Blood 102(11 pt 1):180, 2003.
19.
Badros A: Phase II study of oblimersen,thalidomide, and dexamethasone in patients withrelapsed or refractory multiple myeloma (ProtocolIDs MSGCC-210421, NCI-5824). Availableat http://cancer.gov/search/clinical_trials/results_clinicaltrialsadvanced.aspx. AccessedMay 13, 2004.
20.
Badros AZ, Ratterree B, Natt S, et al:Phase I/II trial of oblimersen sodium (G3139),dexamethasone (Dex) and thalidomide (Thal)in patients with relapsed multiple myeloma (abstract2533). Blood 102:684, 2003.
21.
van de Donk NWCJ, de Weerdt O,Veth G, et al: G3139, a Bcl-2 antisenseoligodeoxynucleotide, induces clinical responsesin VAD-refractory myeloma (abstract2556). Blood 102(11 pt 1):690, 2003.