Targeting the Proapoptotic Factor Bcl-2 in Non-Hodgkin's Lymphoma
Bcl-2 functions as a key survival factor for lymphocytes and is highlyexpressed in a majority of non-Hodgkin's lymphomas. The ability ofoblimersen sodium (Genasense, previously known as G3139) to targetbcl-2 messenger RNA and decrease Bcl-2 protein levels has the potentialto enhance the activity of cytotoxic chemotherapy. Pretreatmentwith oblimersen followed by cyclophosphamide (Cytoxan, Neosar)markedly improved survival relative to single-agent cyclophosphamidein a murine xenograft model. Oblimersen has also enhanced the cytotoxicityof a variety of other agents against non-Hodgkin's lymphoma,including etoposide, rituximab (Rituxan), and alemtuzumab (Campath).An initial phase I study of oblimersen in non-Hodgkin's lymphomademonstrated modest single-agent activity. Recent reports suggest thatoblimersen may add to the activity of R-CHOP (rituximab-cyclophosphamide/doxorubicin/vincristine/prednisone) in previously untreatedmantle cell lymphoma and to rituximab alone in a variety of subtypesof relapsed non-Hodgkin's lymphoma. Additional studies in both treatment-naive and relapsed patients will define the role of oblimersen inthe treatment of non-Hodgkin's lymphoma.
ABSTRACT: Bcl-2 functions as a key survival factor for lymphocytes and is highlyexpressed in a majority of non-Hodgkin's lymphomas. The ability ofoblimersen sodium (Genasense, previously known as G3139) to targetbcl-2 messenger RNA and decrease Bcl-2 protein levels has the potentialto enhance the activity of cytotoxic chemotherapy. Pretreatmentwith oblimersen followed by cyclophosphamide (Cytoxan, Neosar)markedly improved survival relative to single-agent cyclophosphamidein a murine xenograft model. Oblimersen has also enhanced the cytotoxicityof a variety of other agents against non-Hodgkin's lymphoma,including etoposide, rituximab (Rituxan), and alemtuzumab (Campath).An initial phase I study of oblimersen in non-Hodgkin's lymphomademonstrated modest single-agent activity. Recent reports suggest thatoblimersen may add to the activity of R-CHOP (rituximab-cyclophosphamide/doxorubicin/vincristine/prednisone) in previously untreatedmantle cell lymphoma and to rituximab alone in a variety of subtypesof relapsed non-Hodgkin's lymphoma. Additional studies in both treatment-naive and relapsed patients will define the role of oblimersen inthe treatment of non-Hodgkin's lymphoma.
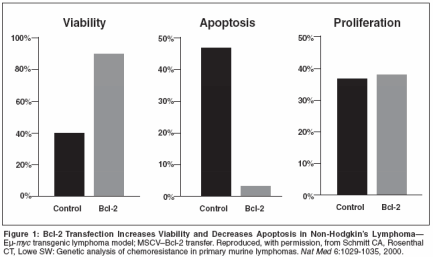
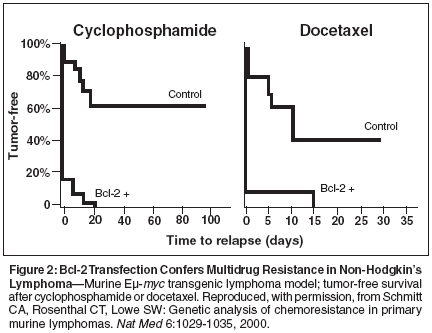
Bcl-2, a member of a family ofproteins primarily located betweenthe two layers of themitochondrial membrane,[1] has beenidentified as a logical therapeutictarget in a variety of human cancers,given its role in regulating a majorapoptotic pathway, namely, themitochondria-mediated or intrinsicpathway of caspase activation.[2,3]Non-Hodgkin's lymphomas of B-cellphenotype are among the malignanciesassociated with a high level ofBcl-2 expression, seen in greater than90% of follicular or mantle cell histologies[4,5] and in 50% of diffuselarge-cell lymphomas.[6-8]No curative therapy exists for follicularand mantle cell lymphomas,and only 50% of all patients presentingwith diffuse large B-cell lymphomawill be long-term failure-freesurvivors. Therefore, strategies thatmay reduce Bcl-2 expression in patientswith these diseases, which makeup almost two-thirds of all non-Hodgkin's lymphomas, are beingevaluated for their potential to reduceresistance to therapy and improve patientoutcomes.Bcl-2 Expression andChemotherapy Drug Resistancein NHLA number of research groups haveindependently found that Bcl-2overexpression is unequivocally associatedwith (1) more advanced disease,according to Ann Arbor criteria; (2) aconstellation of poor prognostic features,as defined by the InternationalPrognostic Index; and (3) significantlylower survival rates.[6-12] The studiesin diffuse large B-cell lymphomahave implicated it as an independentprognostic factor. For example, in aBritish Columbia Cancer Agencystudy of 116 patients with uniformlystaged and treated diffuse large B-celllymphoma, the 8-year overall, diseasefree,and relapse-free survival rateswere significantly (P < .01) lower inpatients whose tumors expressed Bcl-2 protein on initial biopsy: 34%, 32%,and 25% vs 60%, 66%, and 59%, respectively,for those with Bcl-2-negativedisease.[6]Does the expression of Bcl-2 havea biologic impact on malignant cells?In an elegant series of experiments, inwhich lymphoma cells isolated fromEμ-myc transgenic mice wereretrovirally transfected with the murinebcl-2 gene, Schmitt and colleagues[13] attempted to isolate theeffect of a single-gene change on tumorbehavior. Essentially, it was demonstratedthat upregulating Bcl-2 resultsin greater cell viability and lessapoptosis but no difference in the actualrate of proliferation (Figure 1),confirming that the effects are specificto the rate of cellular death. Thisupregulation of Bcl-2 conferred relativeresistance to both cyclophosphamide(Cytoxan, Neosar) and docetaxel(Taxotere), with notable differences inthe rates of tumor-free survival betweenanimals bearing Bcl-2-positivevs Bcl-2-negative lymphomas (Figure 2).
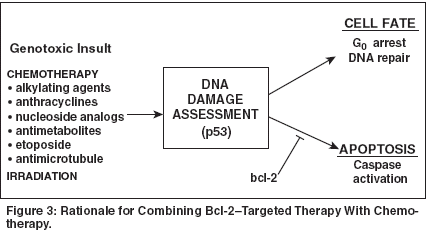
This direct experimental evidence,taken together with the clinical dataamassed over the past decade, makesit clear that strategies designed to decreaseBcl-2 expression in lymphomasand other malignancies have the potentialto alter outcomes significantly.Oblimersen Sodium AntisenseOligonucleotide Therapy inLymphomaOblimersen sodium (Genasense),formerly known as G3139, is an 18-merDNA oligonucleotide that is complementaryto, and thus selectively targets,the first six codons of the bcl-2 messengerRNA (mRNA) to decrease Bcl-2 protein translation. The reverse orientationof the nucleotides to the "sense"strand of the RNA message led to theantisense designation. A sulfa group,added via a phosphorothioate linkage,protects the molecule from degradationby endonucleases and exonucleases,allowing the formulation of a drug witha favorable pharmacokinetic profilewhen administered systemically.The antisense molecules are believedto function as "therapeuticDNA" in that they bind to bcl-2 mRNAin the cytoplasm to form a heteroduplexby Watson-Crick base pairing,thereby creating a biologically undesirablecomplex.[14,15] Various endonucleases,particularly RNase H, aresubsequently engaged to cleave themRNA portion off the DNA backbone,thus eliminating the message whileleaving an intact antisense moleculethat, in theory, can proceed to targetadditional bcl-2 mRNA. In this way,the normal process by which the DNAcode is transcribed into a mRNA thattranslocates into the cytoplasm, bindsa ribosome, and is translated into afunctional Bcl-2 protein is abrogated.Can Bcl-2 actually be demonstrablydownregulated in this way? Workingwith tumor cell lines, numerousgroups, including our own with lymphoidtumors, have shown specificdownregulation of Bcl-2 protein usingoblimersen.[16] Murine SCID/humantumor xenograft models have allowedstudy in vivo that at least mimics theclinical therapeutic situation. In solidtumorstudies that examined humanmelanoma-bearing mice in a subcutaneousmodel, Jansen and colleagues[17], from the University ofVienna, showed that systemically deliveredoblimersen is clearly capableof downregulating Bcl-2 and improvingsensitivity to a cytotoxic agent,dacarbazine (DTIC-Dome).In studies in our laboratory,oblimersen alone, somewhat to oursurprise, completely eliminatedDoHH2 (a transformed follicular lymphomacell line associated with highlevelBcl-2 expression) in a fractionof animals with systemic disease. Reversepolarity oligonucleotides used ascontrols had no effect, a proof in principlethat such widespread diseasecould be targeted by oblimersen.[16]Guinness and coworkers,[18] fromthe Yale Comprehensive CancerCenter, examined the effect ofoblimersen as a single-agent modalityin a human/SCID model of Epstein-Barr virus-associated posttransplantlymphoproliferative disease. In twoseparate in vivo experiments, the administrationof oblimersen (10 mg/m2/d) for 12 days led to a significantlylonger disease-free survival than thatseen in the control group (P < .001),with no evidence of disease in mostoblimersen-treated animals at the timeof sacrifice.However, given what is known aboutthe role of Bcl-2 in preventing the engagementof the cell's apoptotic program,it stood to reason on theoreticalgrounds that Bcl-2 blockade in the faceof the genotoxic insult associated withthe types of therapy customarily givenin clinical practice might yield the greatestbenefit (Figure 3). Most chemotherapeuticagents are known to cause DNAdamage, which is sensed via p53 andother mechanisms, in turn, forcingmalignant cells toward a critical decisionpoint-to activate either repair orcell death programs. A reduction in Bcl-2 expression at this critical momentcould shift the balance in favor of proceedingdown the apoptotic pathway.
Preclinical InvestigationsCombining Oblimersen WithChemotherapy
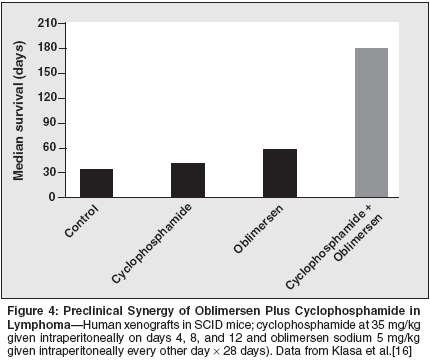
In our own studies at the BritishColumbia Cancer Agency, oblimersengiven as a single agent was shown toimprove the median survival and the90-day survival rate in the SCID xenograftmodel of DoHH2.[16] At alldose levels evaluated, ranging from2.5 mg/kg every other day to 12.5 mg/kg/d, oblimersen-treated animals hada highly significant (P < .000001) survivaladvantage over the untreated andirrelevant oligonucleotide controlgroups. Cyclophosphamide, given asa single agent at a dose of 35 mg/kgon days 4, 8, and 12, exerted modesteffects, improving median survival to47 days from 33 days in the controlgroup, but with no cured animals seen(Figure 4). When oblimersen wascombined with cyclophosphamide ina variety of doses and schedules, thevast majority of animals were cured.Perhaps the most striking findingwas that adding a 28-day course ofoblimersen (2.5 or 5 mg/kg every otherday, a total of 14 doses) to a completelyineffective single-agent cyclophosphamidedose (15 mg/kg on days4, 8, and 12) dramatically increasedthe 90-day survival rate from 0% to50%. The specificity (use of a numberof control oligonucleotides), consistency(across a large number ofanimal cohorts studied), and durability(cured animals were human Bcl-2negative by polymerase chain reactionat necropsy) of this effect provided thescientific rationale for the design ofsubsequent clinical trials.In both preclinical and clinical settings,there has been interest in combiningoblimersen with other agents,including the CD20-targeted monoclonalantibody rituximab (Rituxan).In an in vitro study by Auer et al, exposingprimary chronic lymphocyticleukemia (CLL) cells to the combinationof oblimersen plus rituximab dramaticallyincreased the degree ofapoptosis in a synergistic, dose-depen-dent (for rituximab) manner.[19]
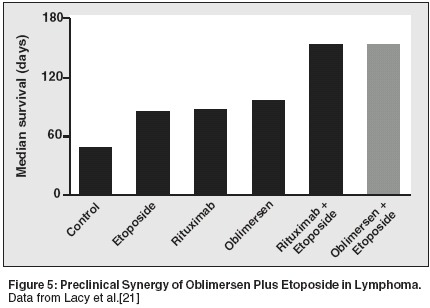
Lacy and collaborators from YaleUniversity[20] reported that combiningoblimersen with rituximab in ahuman/SCID model of Epstein-Barrvirus-associated posttransplantlymphoproliferative disease increasescure rates and significantly prolongssurvival compared with either agentalone. When combining the results oftwo identical experiments, the tumorfreesurvival rates were 79% (11/14)for the combination of oblimersen/rituximab, vs 7% (1/14) and 15%(2/13) for single-agent rituximab andoblimersen, respectively.Expanding on that work, this researchteam showed that oblimersenenhances the in vitro and in vivocytotoxicity of etoposide in thismodel.[21] Oblimersen with etoposidewas associated with a significantlyprolonged median survival of > 150days, vs 98 days for oblimersen aloneand 86 days for etoposide alone (Figure5).As one further example of thesynergistic interaction betweenoblimersen and other targeted therapies,Cotter and colleagues[22] fromBarts and the London School of Medicinefound that the combination ofoblimersen plus alemtuzumab(Campath) exerts more proapoptoticactivity than oblimersen alone in CLLand non-Hodgkin's lymphoma celllines. Collectively, in vitro and in vivopreclinical data support thatoblimersen has a direct effect ondownregulating Bcl-2 and that thiseffect has considerable therapeuticpotential in treating lymphomas.Clinical Studies of Single-Agentand Combination Therapy
To date, clinical data on oblimersenin lymphomas are available from onecompleted phase I trial of single-agenttherapy and two ongoing phase II trialsof rituximab-containing combinationregimens (Table 1).[23-27] Lymphomasare arguably the most logicaltarget for developing oblimersen; however,until recently, clinical researchefforts in hematologic malignancieshave been slow to develop relative tosolid tumors.
- Phase I Trial of Single-AgentOblimersen in Heavily PretreatedLymphoma-In 1997 and 2000, Waters,Webb, and colleagues[26,27]from the United Kingdom publishedthe results of a phase I trial ofoblimersen in 21 patients with varioustypes of previously treated non-Hodgkin's lymphoma, all of whomhad documented Bcl-2 protein expressionby tumor biopsy. Oblimersen wasdelivered as a 14-day continuous subcutaneousinfusion with eight doses,ranging from 4.6 to 195.8 mg/m2/d.Three patients received a second cycle.Most patients had indolent lymphomas(nine with follicular and eightwith small lymphocytic histology) andhad received a median of four chemotherapyregimens.Toxicities generally were mild tomodest, with no significant grade3/4 toxicity up to 110 mg/m2/d.Reversible grade 2/3 thrombocytope-icityin three of five patients treated atthe maximum tolerated dose of147 mg/m2/d, which is equivalent to4 mg/kg/d. In one patient treated at themaximum tolerated dose, who had anunexpectedly high plasma level ofoblimersen, dose-limiting toxicityconsisted of grade 3 fever in conjunctionwith grade 3 hypotension (it resolvedrapidly and did not recur onretreatment at a lower dose).At doses exceeding 37 mg/m2/d,10 patients developed transient grade3/4 lymphopenia; of note, most ofthese patients had preexisting lymphopenia,and any worsening that occurredduring oblimersen therapy wasdeemed clinically insignificant. Grade1/2 hyperglycemia in the nonfastingstate was transiently seen in 19 patients,possibly associated with thesulfur molecule that is part of thephosphorothioate backbone ofoblimersen.One patient with node-positivefollicular lymphoma with bone marrowinvolvement achieved a completeresponse of longer than3 years' duration. Additionally, inthis heavily pretreated population,there were two minor responses andnine patients with stable disease.Disease-related symptoms improvedin 6 of 10 patients (60%) who weresymptomatic prior to treatment, andBcl-2 protein expression decreasedin 7 of 16 (44%) assessable biopsysamples. After a median follow-upof nearly 1 year, median overall anddisease progression-free survivaltimes were 13.4 and 3.6 months, respectively.The feasibility, tolerability, and activityof oblimersen in this study haveprompted investigators to initiatephase II trials of oblimersen as a componentof cytotoxic therapy for bothrelapsed and untreated non-Hodgkin'slymphoma.
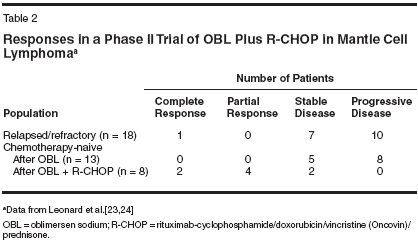
- Phase I/II Trial of OblimersenPlus Rituximab-Cyclophosphamide/Doxorubicin/Vincristine/Prednisonein Mantle Cell Lymphoma-Preclinicalwork within our own group, characterizingmantle cell lymphoma celllines and exposing them to variousagents in vitro and in murine xenograftmodels, indicates that both rituximaband oblimersen are active agents withpotentially additive/synergistic effects.[28,29] Leonard and colleagues[23,24] designed a multicenterphase I/II study of oblimersen in combinationwith rituximab-cyclophosphamide/doxorubicin HCl/vincristine(Oncovin)/prednisone (R-CHOP) inmantle cell lymphoma, a disease witha median survival of only 3 to 4 yearsassociated with overexpression ofboth Bcl-1 and Bcl-2, the latter ofwhich has been somewhat underappreciated.Treatment was initiated withoblimersen (3 to 5 mg/kg/d) administeredas a 7-day continuous infusionevery 21 days for up to six cycles oruntil disease progression; treating relapsed/refractory patients with an additionalsix cycles was permitted. Inchemotherapy-naive patients failingto achieve a complete response aftersix cycles of oblimersen alone, subsequenttherapy consisted ofoblimersen (3 mg/m2/d) on days 1 to7 plus R-CHOP on day 5 for up to sixcycles.Results for the first 45 enrolled patients,27 with relapsed/refractory and18 with chemotherapy-naive disease,have been reported in abstract form(Table 2). All six cycles of initialoblimersen therapy were completedwithout evidence of disease progressionin seven relapsed/refractory andtwo chemotherapy-naive patients. Of18 patients with relapsed/refractorydisease treated at the 3-mg/kg/d dose(determined to be the maximum tolerateddose for cycle 1), 1 patientachieved a complete response and7 patients had stable disease. No objectiveresponses to single-agentoblimersen were seen among the13 evaluable patients in the chemotherapy-naive subset. However, six ofeight evaluable patients had a completeresponse (n = 2) or partial response(n = 4) on completion ofoblimersen plus R-CHOP, an objectiveresponse rate of 75%.Based on preliminary safety analysis,combination therapy has been welltolerated, and adding oblimersen toR-CHOP did not appear to increase theexpected toxicity of this regimen.
- Phase I/II Trial of Oblimersen PlusRituximab in B-Cell Non-Hodgkin'sLymphoma-With oblimersen andrituximab demonstrating synergyagainst lymphoma in vitro and at leastadditive effects in xenografts, Proand colleagues[25,30] from theUniversity of Texas M.D. AndersonCancer Center initiated a phase II trialof this combination in refractory/recurrent B-cell non-Hodgkin'slymphoma. Oblimersen (3 mg/kg/d)is given as a 7-day continuousintravenous infusion every 2 weeks(on days 1 to 7, 15 to 21, and 29 to35) in conjunction with six doses ofonce-weekly rituximab (375 mg/m2)on days 3, 8, 15, 22, 29, and 36. Inthe absence of disease progression orunacceptable toxicity, one additionalcourse was permitted.
- Most of the first 16 enrolledpatients had indolent histologies, themost common being follicularlymphoma (n = 8), and 14 patients hadpreviously received rituximab. In the11 evaluable patients, four responseswere seen, for a preliminary responserate of 36%. One complete responsewas seen in a patient with stage IVmucosa-associated lymphoid tissuelymphoma with bone marrow involvement.All but one of the respondershad a history of rituximab exposure.Accrual into this trial is ongoing as ofMarch 2004, with a target enrollmentof 120 patients.
Conclusion
Bcl-2 appears to be a keyantiapoptotic factor in non-Hodgkin'slymphoma. Oblimersen has demonstratedmodest single-agent activityagainst various types of non-Hodgkin's lymphoma, and clinicalresearch efforts are seeking to combinethis antisense oligonucleotidewith other forms of effective therapy.The maximum tolerated dose foroblimersen has been 3 mg/kg/d in patientswith B-cell hematologic malignanciesvs 7 mg/m
2
/d in patients withsolid tumors.For reasons that are not yet fullyunderstood, patients with non-Hodgkin's lymphoma and CLL appearto be more susceptible todeveloping certain types of toxicities(particularly hypotension and fever)during oblimersen therapy than thosewho have solid tumors. Accumulatingpreclinical data support thechemosensitizing potential ofoblimersen, and that it can be safelyadministered with chemotherapeuticagents, singly and in combination, aswell as with other targeted therapiessuch as rituximab.A number of studies combiningoblimersen with various chemotherapycombinations or single drugsin a variety of histologic subtypes ofnon-Hodgkin's lymphoma are inprogress. These phase II and III studies,modeled on the preclinical work,will determine whether the addition ofan antiapoptotic agent to a variety ofestablished as well as novel approachesused in treating non-Hodgkin's lymphoma can increasetheir clinical efficacy.
Disclosures:
Dr. Klasa has consultedfor and is a member of the speakers bureaus ofOrtho-McNeil Pharmaceuticals, Eli Lilly andCompany, Berlex Laboratories, AventisPharmaceuticals, Genta Incorporated, andSchering AG.
References:
1.
Krajewski S, Tanaka S, Takayama S, etal: Investigation of the subcellular distributionof the bcl-2 oncoprotein: Residence in thenuclear envelope, endoplasmic reticulum, andouter mitochondrial membranes. Cancer Res53:4701-4714, 1993.
2.
Klasa RJ, Gillum AM, Klem RE, et al:Oblimersen Bcl-2 antisense: Facilitatingapoptosis in anticancer treatment. AntisenseNucleic Acid Drug Dev 12:193-213, 2002.
3.
Korsmeyer SJ: BCL-2 gene family and theregulation of programmed cell death. CancerRes 59(suppl):1693S-1700S, 1999.
4.
Gaulard P, d’Agay MF, Peuchmaur M, etal: Expression of the bcl-2 gene product in follicularlymphoma. Am J Pathol 140:1089-1095,1992.
5.
Pezzella F, Tse AG, Cordell JL, et al: Expressionof the bcl-2 oncogene protein is notspecific for the 14;18 chromosomal translocation.Am J Pathol 137:225-232, 1990.
6.
Gascoyne RD, Adomat SA, Krajewski S,et al: Prognostic significance of Bcl-2 proteinexpression and Bcl-2 gene rearrangement indiffuse aggressive non-Hodgkin’s lymphoma.Blood 90:244-251, 1997.
7.
Hermine O, Haioun C, Lepage E, et al forthe Groupe d’Etude des Lymphomes del’Adulte (GELA): Prognostic significance ofbcl-2 protein expression in aggressive non-Hodgkin’s lymphoma. Blood 87:265-272,1996.
8.
Hill ME, MacLennan KA, CunninghamDC, et al: Prognostic significance of BCL-2expression and bcl-2 major breakpoint regionrearrangement in diffuse large cell non-Hodgkin’s lymphoma: A British National LymphomaInvestigation Study. Blood 88:1046-1051, 1996.
9.
Barrans SL, Carter I, Owen RG, et al:Germinal center phenotype and bcl-2 expressioncombined with the International PrognosticIndex improves patient risk stratification indiffuse large B-cell lymphoma. Blood 99:1136-1143, 2002.
10.
Colomo L, López-Guillermo A, PeralesM, et al: Clinical impact of the differentiationprofile assessed by immunophenotyping inpatients with diffuse large B-cell lymphoma.Blood 101:78-84, 2003.
11.
de Jong D, Glas AM, Boerrigter L, et al:Very late relapse in diffuse large B-cell lymphomarepresents clonally related disease andis marked by germinal center cell features.Blood 102:324-327, 2003.
12.
Mounier N, Briere J, Gisselbrecht C, etal: Rituximab plus CHOP (R-CHOP) overcomesbcl-2-associated resistance to chemotherapyin elderly patients with diffuse largeB-cell lymphoma (DLBCL). Blood 101:4279-4284, 2003.
13.
Schmitt CA, Rosenthal CT, Lowe SW:Genetic analysis of chemoresistance in primarymurine lymphomas. Nat Med 6:1029-1035,2000.
14.
Stephenson ML, Zamecnik PC: Inhibitionof Rous sarcoma viral RNA translation bya specific oligodeoxyribonucleotide. Proc NatlAcad Sci U S A 75:285-288, 1978.
15.
Zamecnik PC, Stephenson ML: Inhibitionof Rous sarcoma virus replication and celltransformation by a specific oligodeoxynucleotide.Proc Natl Acad Sci U S A 75:280-284, 1978.
16.
Klasa RJ, Bally MB, Ng R, et al: Eradicationof human non-Hodgkin’s lymphoma inSCID mice by BCL-2 antisense oligonucleotidescombined with low-dose cyclophosphamide.Clin Cancer Res 6:2492-2500, 2000.
17.
Jansen B, Schlagbauer-Wadl H, BrownBD, et al: Bcl-2 antisense therapy chemosensitizeshuman melanoma in SCID mice. NatMed 4:232-234, 1998.
18.
Guinness ME, Kenney JL, Reiss M, etal: Bcl-2 antisense oligodeoxynucleotidetherapy of Epstein-Barr virus-associatedlymphoproliferative disease in severe combinedimmunodeficient mice. Cancer Res 60:5354-5358, 2000.
19.
Auer RL, Corbo M, Fegan CD, et al: Bcl-2 antisense (Genasenseâ¢) induces apoptosisand potentiates activity of both cytotoxic chemotherapyand rituximab in primary CLL cells(abstract 3358). Blood 98(suppl 1):808, 2001.
20.
Loomis R, Carbone R, Reiss M, et al:Bcl-2 antisense (G3139, Genasense) enhancesthe in vitro and in vivo response of Epstein-Barr virus-associated lymphoproliferative diseaseto rituximab. Clin Cancer Res 9:1931-1939, 2003.
21.
Lacy J, Loomis R: Bcl-2 antisense(G3139, oblimersen) and rituximab enhancechemosensitivity of EBV-associatedlymphoproliferative disease in vitro and in vivo(abstract 863). Proc Am Soc Clin Oncol 22:215,2003.
22.
Cotter FE, Auer R, Corbo M, et al:Oblimersen sodium (G3139) sensitizes malignantB-cells to alemtuzumab (Ab) inducedapoptosis (abstract 910). Proc Am Soc ClinOncol 22:227, 2003.
23.
Leonard JP, Coleman M, Vose J, et al:Phase II study of oblimersen sodium (G3139)alone and with R-CHOP in mantle cell lymphoma(MCL) (abstract 2276). Proc Am SocClin Oncol 22:566, 2003.
24.
Leonard JP, Hainsworth J, Bernstein S,et al: Genasense (oblimersen sodium, G3139)is active and well-tolerated both alone and withR-CHOP in mantle cell lymphoma (MCL) (abstract490). Blood 102:143, 2003.
25.
Pro B, Smith MR, Younes A, et al:Genasense (Bcl-2 antisense) plus rituximab isactive in patients with relapsed or refractoryB-cell non-Hodgkin’s lymphoma (abstract1491). Blood 102:410, 2003.
26.
Waters JS, Webb A, Cunningham D, etal: Phase I clinical and pharmacokinetic studyof bcl-2 antisense oligonucleotide therapy inpatients with non-Hodgkin’s lymphoma. J ClinOncol 18:1812-1823, 2000.
27.
Webb A, Cunningham D, Cotter F, et al:BCL-2 antisense therapy in patients with non-Hodgkin lymphoma. Lancet 349:1137-1141,1997.
28.
Bebb DG, Tucker C, Williams ME, etal: Effect of cyclophosphamide, BCL2antisense oligonucleotides and rituximab onmantle cell lymphoma growth in a murine invivo model (abstract 1372). Blood 100(11 pt1):354, 2002.
29.
Williams ME, Bebb DG, Tucker C, etal: Mantle cell lymphoma: In vitro and murinepreclinical models utilizing five humancell lines (abstract 1378). Blood 100(11 pt1):355, 2002.
30.
Pro B: Phase II study of oblimersenand rituximab in patients with recurrentB-cell non-Hodgkin’s lymphoma. ProtocolMDA-ID-02148, NCI-5808. Availableat:http://cancer.gov/clinicaltrials/view_c l i n i c a l t r i a l s . a s p x ? v e r s i o n =healthprofessional&cd rid=270426&protocolsearchid=842445. Accessed March26, 2004.