Potential Therapeutic Applications of Oblimersen in CLL
Bcl-2 protein is upregulated in a wide variety of lymphoid malignancies,including chronic lymphocytic leukemia (CLL). The proteinis thought to be responsible for maintaining the viability of malignantlymphoid cells and may contribute to chemotherapy and radiotherapyresistance. Previous studies have shown that reduction of bcl-2 expressionby antisense therapy sensitizes cells to chemotherapy-inducedapoptosis. In vitro, the Bcl-2 antisense drug oblimersen sodium(Genasense, previously known as G3139) enhances the apoptotic responsein CLL cells to fludarabine (Fludara), corticosteroids,alemtuzumab (Campath), and rituximab (Rituxan). A phase I trial inpatients with refractory/relapsed CLL showed that patients with CLLwere more sensitive to oblimersen than patients with solid tumors. Themaximum tolerated oblimersen dose was 3 mg/kg/d, and the most commondose-limiting reaction was hypotension, frequently in associationwith high spiking fever. In this study, oblimersen displayed limited singleagentactivity, including tumor lysis syndrome, transient decreases incirculating CLL cells, and reduction of splenomegaly and size of lymphnodes. Major responses were observed in 9% of patients. Subsequently,a phase III trial evaluating fludarabine and cyclophosphamide with orwithout oblimersen (3 mg/kg/d for 7 days) was initiated in patients withrelapsed or refractory CLL. This trial recently completed accrual of241 patients.
ABSTRACT: Bcl-2 protein is upregulated in a wide variety of lymphoid malignancies,including chronic lymphocytic leukemia (CLL). The proteinis thought to be responsible for maintaining the viability of malignantlymphoid cells and may contribute to chemotherapy and radiotherapyresistance. Previous studies have shown that reduction of bcl-2 expressionby antisense therapy sensitizes cells to chemotherapy-inducedapoptosis. In vitro, the Bcl-2 antisense drug oblimersen sodium(Genasense, previously known as G3139) enhances the apoptotic responsein CLL cells to fludarabine (Fludara), corticosteroids,alemtuzumab (Campath), and rituximab (Rituxan). A phase I trial inpatients with refractory/relapsed CLL showed that patients with CLLwere more sensitive to oblimersen than patients with solid tumors. Themaximum tolerated oblimersen dose was 3 mg/kg/d, and the most commondose-limiting reaction was hypotension, frequently in associationwith high spiking fever. In this study, oblimersen displayed limited singleagentactivity, including tumor lysis syndrome, transient decreases incirculating CLL cells, and reduction of splenomegaly and size of lymphnodes. Major responses were observed in 9% of patients. Subsequently,a phase III trial evaluating fludarabine and cyclophosphamide with orwithout oblimersen (3 mg/kg/d for 7 days) was initiated in patients withrelapsed or refractory CLL. This trial recently completed accrual of241 patients.
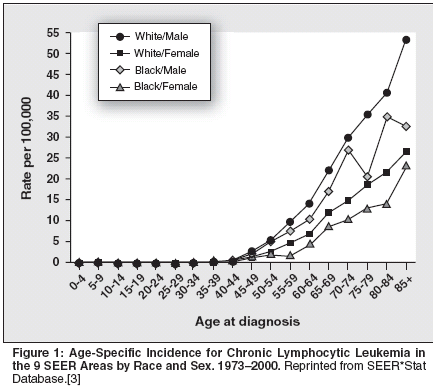
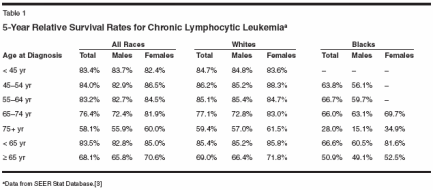
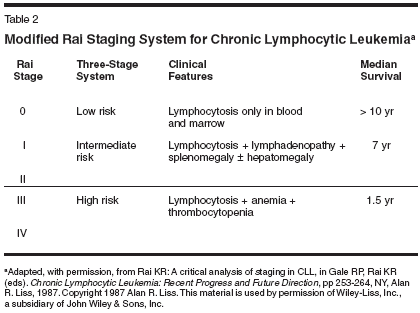
B-cell chronic lymphocytic leukemia(B-CLL) is a disordercharacterized by the unremittingaccumulation of small, slowlyproliferating CD5+/CD19+/CD20+(weak)/CD23+/sIg+ (weak) monoclonalB cells.[1]CLL is very rare in people youngerthan age 40. However, its incidence increasessharply after the fourth decadeof life, to the point of being the predominantleukemic type in the elderly in theWestern world, across race and sex differences(Figure 1). It is estimated that7,300 new cases were diagnosed in theUnited States in 2003.[2] Statistics fromthe nine population-based cancer registriesof the National Cancer Institute'sSurveillance, Epidemiology and EndResults (SEER) Program for 2000showed significantly better survival differencesfor patients younger than age65 (average presentation age: 64 years)than for those 65 years of age and older,although CLL shortens life expectancyconsiderably, even in younger patients(Table 1).[3]Prognostic IndicatorsNeither the Rai[4] nor the Binet[5]staging systems, which discriminateCLL by the sites of disease and/or thedegree of cytopenias induced by leukemicmarrow replacement, enablephysicians to identify patients in thegood-prognosis group who will eventuallyprogress. However, over time,these systems have proved to be extremelyuseful for categorizing patients,particularly those participatingin clinical trials.The original Rai system[4] consistedof five stages (0 to IV) but wasmodified to a three-stage scheme in1987,[6] when analysis of survivaldata in a large number of patients establishedthree distinct risk categories(low, intermediate, and high), whichdiffer significantly in terms of mediansurvival. Patients in the low-risk group(stage 0) have lymphocytosis withno other abnormality; patients in theintermediate-risk group have enlargedlymph nodes (stage I) and/or spleen(stage II) in addition to lymphocytosis;and patients in the high-riskgroup have anemia (hemoglobin value< 11.0 g/dL, stage III) and/or thrombocytopenia(platelet count of < 100 *109/L, stage IV) (Table 2).Serum levels of beta2-microglobulin,[7] lactate dehydrogenase,soluble CD23,[8] and the cell-surfaceexpression of CD38[9] can help predictdisease activity. However, the presenceof certain cytogenetic abnormalities inthe leukemic B cells [10,11] and/or somaticmutations in the immunoglobulinheavy-chain genes seem to predictrapid disease progression and survivalmore accurately.[12,13]Genetic ComponentsCLL B cells with mutated immunoglobulinheavy-chain genes are associatedwith the more favorablegenetic defect deletion 13q14. Conversely,unmutated immunoglobulinheavy-chain genes are associated withtrisomy 12 and the high-risk 17p and11q genomic aberrations.[14] CLL Bcells with unmutated immunoglobulinheavy-chain genes generally showthe distinctive expression of the zetaassociatedprotein of 70 kD (ZAP-70),an intracellular tyrosine kinase, whichhas been found to be associated withenhanced immunoglobulin receptorsignaling in CLL B cells. Mountingdata indicate that expression of ZAP-70 is a more reliable marker of the riskof early disease progression than themutational status of the expressed immunoglobulinheavy-chain genes inCLL.[15,16]
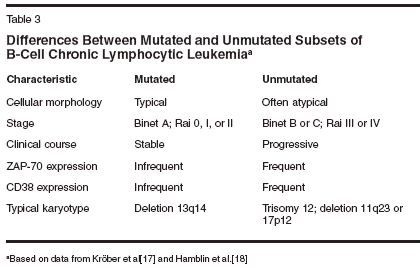
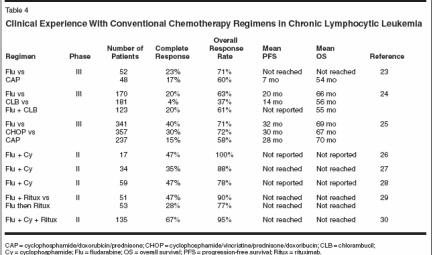
Disease SubtypesBased on these recent findings, ithas been proposed that there are twotypes of CLL (Table 3). The first typearises from relatively less differentiatedor "immunologically naive"pregerminal B cells with unmutatedimmunoglobulin heavy-chain genes,displays atypical CLL morphology,and has a poor prognosis. The othertype evolves from more differentiatedpostgerminal "memory" B cells withsomatically mutated immunoglobulinheavy-chain genes and has a goodprognosis.[17,18]Treatment With Chemotherapyor ChemoimmunotherapyApproximately one-third of patientswith CLL never require treatment. Inanother third, an initial indolent phaseis followed by disease progression. Theremaining third show aggressive diseaseat the outset and need immediate treatment.Ultimately, 50% of patients withCLL will require treatment. Deferringtherapy until progression of CLL doesnot affect survival. Neither single-agentchlorambucil (Leukeran) nor a varietyof combination chemotherapy regimens(some including anthracyclines) havebeen shown to increase response ratesor improve survival in clinical studies.[19-21] A meta-analysis of 2,048patients with early-stage disease includedin seven randomized trials comparingimmediate or deferred treatmentwith chlorambucil demonstrated nobenefit in either arm.[22] A summaryof clinical experience with chemotherapyand chemoimmunotherapy inCLL is provided in Table 4.[23-30]
Fludarabine (Fludara), a purineanalog, yields better response ratesthan chlorambucil but causes moremyelosuppression and greater reductionin CD4+ lymphocytes.[31] Theresults of three phase III randomizedtrials in previously untreated patientswith CLL demonstrated the superiorityof fludarabine over other alkylatingagent-based therapy in terms ofresponse, duration of remission, anddisease progression-free survival (butnot overall survival).[24,25,27]A phase II trial conducted by Flinnand colleagues[26] showed a completeresponse rate of 47% in 17 untreatedpatients with CLL who receivedfludarabine and cyclophosphamideplus filgrastim (Neupogen) support.In a subsequent randomizedphase II trial that was conducted forthe Cancer and Leukemia Group B(CALGB 9712), Byrd et al[29] demonstratedthat the administration offludarabine concurrently withrituximab (Rituxan) for 6 monthlycourses followed by rituximab2 months later for 4 weekly coursesalso produced a complete responserate of 47% in 51 untreated patientswith B-CLL. These results comparedfavorably with results thatwere observed in 53 patients whoreceived fludarabine alone for6 months followed sequentially2 months later by rituximab consolidation.[29]Of note, two studies[32,33] testinghigher doses of single-agent rituximabin patients with pretreated and untreatedB-CLL demonstrated a doseresponserelationship for this agent. Inaddition, investigators at the Universityof Texas M. D. Anderson CancerCenter recently reported a completeresponse rate of 67% in 135 patientswith CLL receiving fludarabine (25mg/m2/d) for 3 days, cyclophosphamide(250 mg/m2/d) for 3 days, andrituximab (375 to 500 mg/m2) on day1 of each treatment cycle.[30] Theseresponses included a high proportionof patients with molecular remissions,indicating the possibility to consolidatethe response with autologoushematopoietic cell transplantation.Another treatment option availablefor patients with chronic lymphocyticleukemia is conventional allogeneicbone marrow transplantation, whichmay be curative in some cases.However, only 10% of patients are eligiblefor this treatment, which is associatedwith significant morbidityand mortality.[34] Allogeneic bonemarrow transplantation in which thepatient's marrow is not completely ablatedby chemotherapy with or withoutlow-dose radiotherapy (oftentermed a minitransplant) is anotheroption now being evaluated for patientswith CLL.[35]
Treatment With OblimersenSodiumPreclinical Experience
A variety of investigational agentsthat include an assortment of chemicalcompounds, monoclonal antibodies,and biologic approaches have beenevaluated for the treatment of CLL andawait confirmation of their clinical usefulnessin comparative studies (Table 5).Notable among them is oblimersen sodiuminjection (Genasense, formerlyknown as G3139), an innovative treatmentmodality in the form of anantisense oligonucleotide targeted to theBcl-2 molecule.Bcl-2 protein is upregulated in a widevariety of lymphoid malignancies, includingCLL. The protein is thought tobe responsible for maintaining the viabilityof malignant lymphoid cells andmay contribute to chemotherapy andradiotherapy resistance.[36,37] Higherlevels of Bcl-2 protein expression havebeen inversely correlated with survivalin previously untreated patients withCLL.[38] In B-CLL cells, expressionlevels of Bcl-2 protein were significantlyelevated over those of normal B cellsand correlated inversely with those ofthe proapoptotic protein BAX.[39] Inaddition, quantitative expression analysisby reverse transcription polymerasechain reaction showed the relative ratioof Bcl-2 protein to BAX to be markedlyincreased in CLL and also inmantle cell lymphoma.[40] Previousstudies have shown that reduction ofBcl-2 protein expression by antisensetherapy sensitizes cells to chemotherapy-induced apoptosis.[41,42]The activity of oblimersen in CLLhas been evaluated in vitro and in vivo.Auer et al[43] showed that in cellsobtained by CD19 selection of peripheralblood samples from patients withCLL, Bcl-2 protein expression wassignificantly downregulated andmarkers of apoptosis upregulated byoblimersen in a sequence-specificmanner, compared with sense and nonsenseoligonucleotide controls. In thissystem, oblimersen (2 μM) was moreactive than either fludarabine (50 μM)or dexamethasone (1 μM) as an inducerof apoptosis, and potentiationof this activity was noted whenoblimersen was combined with eitherfludarabine or dexamethasone.[43]Furthermore, pretreatment withoblimersen sensitized CLL cells to theapoptotic effect of rituximab in a doseresponserelationship.With similar culture systems, synergismto induce apoptosis has beenshown in vitro for oblimersen combinedwith the novel proteasome inhibitorbortezomib (PS-341, Velcade)[44] and with the humanized anti-CD52 monoclonal antibody alemtuzumab(Campath 1H).[45] In thesestudies, CD19 antibody-selected CLLcells from 12 patients were put inshort-term culture with or withoutoblimersen (at a concentration of 5 μM)to reduce Bcl-2 protein levels. Controlsense and nonsense oligonucleotideswere also used. Alemtuzumabwas added at concentrations rangingfrom 1 μg/mL to 10 μg/mL. All CLLsamples showed some apoptosis withalemtuzumab alone; however, thosecells pretreated with oblimersenshowed an enhanced level ofapoptosis. The addition of oblimersengreatly enhanced the effectiveness ofalemtuzumab with similar cell kill at20% of the dose compared withalemtuzumab alone.[45]
Clinical Experience
Oblimersen was evaluated in a nonrandomizedphase I/II trial asmonotherapy for heavily pretreatedpatients with relapsed or refractoryCLL to determine the maximum tolerateddose and evaluate single-agentactivity.[46] Fourteen patients receivedoblimersen as a continuous intravenous(IV) infusion at a daily doseranging from 3 to 7 mg/kg for 5 to 7days every 3 weeks. Several patientsexhibited antitumor effects, includingtumor lysis, reduction in circulatingCLL cells, and decreases in lymphadenopathyand splenomegaly. One patientwith Richter's syndrome developeda stable partial response that wasreported to last several years.In cycle 1, patients received one offour different oblimersen doses: 3, 4,5, or 7 mg/kg/d. Six of the patients receivinghigher dosages (3 receiving7 mg/kg/d, 1 receiving 5 mg/kg/d, and2 receiving 4 mg/kg/d) showed doselimitingtoxicity requiring treatmentdiscontinuation (high fever, severehypotension, hypoglycemia, and backpain requiring opiate analgesics)despite reduction of leukocytosis. Incycle 2, two patients who were escalatedfrom a lower dose to 7 mg/kg/dalso showed dose-limiting toxicity.These findings clearly demonstratedthat patients with CLL are more sensitiveto the side effects of oblimersenthan patients with solid tumors, inwhom these doses are generally welltolerated. Either tumor lysis or directoligonucleotide immunostimulation ofthe malignant B cells is thought to beresponsible for this distinct toxicitypattern.[47,48]In a subsequent phase I/II trial in26 patients with relapsed or refractoryCLL who had previously receivedfludarabine, oblimersen was administeredas a continuous IV infusion at adosage of 3 mg/kg/d for 5 to 7 daysevery 3 to 4 weeks.[49] Six patientsin phase I received the phase II doseof oblimersen (3-4 mg/kg/d). Patientshad received a median of 3 (range: 1-13) prior chemotherapy regimens forCLL. Thirteen, 7, and 4 patients hadRai stages II, III, and IV, respectively,and 2 patients had Richter's transformation.The median age was 61 years(range, 44-70). The evaluable populationincluded 23 patients who receivedthe phase II dose for ≥ 2 cycles(6 enrolled in phase I, and 17 enrolledin phase II).Two patients (9%) achieved partialresponse, 11 (48%) showed stable disease,and the remaining 10 patients(43%) had progressive disease. Reductionsin circulating CLL cells of ≥ 50%from baseline were seen in 9 of 23 patients(39%). In addition ≥ 50% decreasein lymphadenopathy was seen in8 of 19 patients (42%), and a ≥ 50%reduction of hepatomegaly or splenomegalywas seen in 8 of 16 patients (50%).Oblimersen (3 to 4 mg/kg/d) was welltolerated, with only rare grade 3 orhigher toxicities reported. The mostcommon symptoms were fatigue, nightsweats, increased dyspnea, and pneumonia.Results confirmed thatoblimersen has antitumor activity in pa-tients with CLL, even in the absence ofother cytotoxic drugs, and that a dailydose of 3 mg/kg was well tolerated.Subsequently, a phase III trialevaluating fludarabine (25 mg/m2) andcyclophosphamide (250 mg/m2; days1-3) with or without oblimersen(3 mg/kg/d continuous IV infusion;days 1-7) was initiated in patients withrelapsed or refractory CLL. Stratificationat recruitment grouped patientsaccording to three criteria: 1) "responsiveness"or "refractoriness" tofludarabine; 2) number of previouschemotherapy regimens (1-2 vs ≥ 3);and 3) duration of response to priortherapy (≥ 6 vs < 6 months). The primaryend point was response rate(complete response plus nodular partialresponse), and secondary endpoints were overall response (completeresponse plus nodular partial responseplus partial response), overallsurvival, and time to disease progression.This trial recently completedaccrual of 241 patients.ConclusionThe rationale for evaluatingoblimersen in CLL is that Bcl-2 proteinis highly expressed and in all likelihoodis a key survival factor in thisdisease. Oblimersen has shown limitedsingle-agent activity in patientswith previously treated CLL. In addition,patients with CLL appear to haveconsiderably more sensitivity to thedevelopment of significant hypotensionand fever than patients with solidtumors who receive oblimersen atsimilar dosages, who exhibit better tolerance.Oblimersen 3 mg/kg/d waswell tolerated in phase II trials, andthis dosage was used in the phase IIItrial. A trial of oblimersen combinedwith fludarabine and rituximab in patientswith previously untreated or relapsedCLL has recently been initiated.
Disclosures:
The author has nosignificant financial interest or otherrelationship with the manufacturers of anyproducts or providers of any service mentionedin this article.
References:
1.
Cheson BD, Bennett JM, Grever M, et al:National Cancer Institute-Sponsored WorkingGroup Guidelines for Chronic LymphocyticLeukemia: Revised Guidelines for Diagnosisand Treatment. Blood 87:4990-4997, 1996.
2.
Jemal A, Murray T, Samuels A, et al: Cancerstatistics, 2003. CA Cancer J Clin 53:5-26,2003.
3.
Surveillance, Epidemiology, andEnd Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: IncidenceSEER 9 Regs, 11/02 Sub (1973-2000), NCI,DCCPS, Surveillance Research Program, CancerStatistics Branch, rel. 4/03, based on 11/02submission.
4.
Rai KR, Sawitsky A, Cronkite EP, et al:Clinical staging of CLL. Blood 46:219-234,1975.
5.
Binet JL, Leporrier M, Dighiero G, et al:A clinical staging system for chronic lymphocyticleukaemia: Prognostic significance.Cancer 40:855-864, 1977.
6.
Rai KR: A critical analysis of staging inCLL, in Gale RP, Rai KR (eds): Chronic LymphocyticLeukemia: Recent Progress and FutureDirection, pp 253-264. NY, Alan R. Liss, 1987.
7.
Kantarjian HM, Smith T, Estey E, et al:Prognostic significance of elevated serum beta2-microglobulin levels in adult acute lymphocyticleukemia. Am J Med 93:599-604, 1992.
8.
Molica S, Levato D, Dell’Olio M, et al:Cellular expression and serum circulating levelsof CD23 in B-cell chronic lymphocyticleukaemia: Implications for prognosis.Haematologica 81:428-433, 1996.
9.
Ibrahim S, Keating M, Do KA, et al: CD38expression as an important prognostic factorin B-cell chronic lymphocytic leukemia. Blood98:181-186, 2001.
10.
Han T, Ozer H, Sadamori N, et al: Prognosticimportance of cytogenetic abnormalitiesin patients with chronic lymphocytic leukemia.N Engl J Med 310:288-292, 1984.
11.
Juliusson G, Oscier DG, Fitchett M, etal: Prognostic subgroups in B-cell chroniclymphocytic leukemia defined by specificchromosomal abnormalities. N Engl J Med323:720-724, 1990.
12.
Hamblin TJ, Davis Z, Gardiner A, et al:Unmutated Ig V(H) genes are associated witha more aggressive form of chronic lymphocyticleukemia. Blood 94:1848-1854, 1999.
13.
Damle RN, Wasil T, Fais F, et al: Ig Vgene mutation status and CD38 expression asnovel prognostic indicators in chronic lymphocyticleukemia. Blood 94:1840-1847, 1999.
14.
Oscier DG, Gardiner AC, Mould SJ, etal: Multivariate analysis of prognostic factorsin CLL: Clinical stage, IGVH gene mutationalstatus, and loss or mutation of the p53 geneare independent prognostic factors. Blood100:1177-1184, 2002.
15.
Rassenti LZ, Huynh L, Toy TL, et al:ZAP-70 is a more reliable marker of diseaseprogression risk than immunoglobulin mutationstatus in chronic lymphocytic leukemia(abstract 106). Blood 102(11 pt 1):34, 2003.
16.
Grever MR, Dewald GW, Lucas DM, etal: ZAP-70 protein expression varies by interphasecytogenetic group and may predict diseaseprogression to requirement of treatmentamong select genetic groups in patients withchronic lymphocytic leukaemia (CLL) (abstract244). Blood 102(11 pt 1):73, 2003.
17.
Kröber A, Seiler T, Benner A, et al: IgV(H)mutation status, CD38 expression level, genomicaberrations, and survival in chronic lymphocyticleukemia. Blood 100:1410-1416, 2002.
18.
Hamblin TJ, Orchard JA, Ibbotson RE, etal: CD38 expression and immunoglobulin variableregion mutation are independent prognosticvariables in chronic lymphocytic leukemia,but CD38 expression may vary during the courseof the disease. Blood 99:1023-1029, 2002.
19.
French Cooperative Group on ChronicLymphocytic Leukemia: Effects of chlorambuciland therapeutic decision in initial forms of CLL(stage A): Results of a randomized clinical trialon 612 patients. Blood 75:1414-1421, 1990.
20.
French Cooperative Group on ChronicLymphocytic Leukaemia: Long-term resultsof the CHOP regimen in stage C chronic lymphocyticleukemia. Br J Haematol 73:334-340, 1989.
21.
French Cooperative Group on ChronicLymphocytic Leukemia: A randomized clinicaltrial of chlorambucil versus COP in stageB chronic lymphocytic leukemia. Blood75:1422-1425, 1990.
22.
CLL Trialists’ Collaborative Group: Chemotherapeuticoptions in chronic lymphocyticleukemia: A meta-analysis of the randomizedtrials. J Natl Cancer Inst 91:861-868, 1999.
23.
Johnson S, Smith AG, Loffler H, et al:Multicentre prospective randomised trial offludarabine vs cyclophosphamide, doxorubicin,and prednisone for treatment of advancedstagechronic lymphocytic leukaemia. FrenchCooperative Group on CLL. Lancet 347:1432-1438, 1996.
24.
Rai KR, Peterson BL, Appelbaum FR,et al: Fludarabine compared with chlorambucilas primary therapy for chronic lymphocyticleukemia. N Engl J Med 343:1750-1757, 2000.
25.
Leporrier M, Chevret S, Cazin B, et al:Randomized comparison of fludarabine, CAPand CHOP in 938 previously untreated stageB and C chronic lymphocytic leukemia patients.Blood 98:2319-2325, 2001.
26.
Flinn IW, Byrd JC, Morrison C, et al:Fludarabine and cyclophosphamide withfilgrastim support in patients with previouslyuntreated indolent lymphoid malignancies.Blood 96:71-75, 2000.
27.
O'Brien SM, Kantarjian HM, Cortes J, etal: Results of the fludarabine and cyclophosphamidecombination regimen in CLL. J Clin Oncol19:1414-1420, 2001.
28.
Cazin B, Maloum K, Divine M, et al: Oralfludarabine and cyclophosphamide in previouslyuntreated CLL: Preliminary data on 59 pts (abstract3214). Blood 98(11 pt 1):772, 2001.
29.
Byrd JC, Peterson BL, Morrison VA, etal: Randomized phase 2 study of fludarabinewith concurrent versus sequential treatmentwith rituximab in symptomatic, untreated patientswith B-cell chronic lymphocytic leukemia:Results from Cancer and Leukemia GroupB 9712 (CALGB 9712). Blood 101:6-14, 2003.
30.
Keating MJ, Manshouri T, O’Brien S, etal: A high proportion of molecular remissionscan be obtained with a fludarabine, cyclophosphamide,rituximab combination (FCR) inchronic lymphocytic leukaemia (CLL) (abstract771). Blood 100(11 pt 1):205, 2002.
31.
Grever MR, Kopecky KJ, Coltman CA,et al: Fludarabine monophosphate: A potentiallyuseful agent in chronic lymphocytic leukemia.Nouv Rev Fr Hematol 30:457-459, 1988.
32.
O’Brien SM, Kantarjian H, Thomas DA,et al: Rituximab dose-escalation trial in chroniclymphocytic leukemia. J Clin Oncol 19:2165-2170, 2001.
33.
Byrd JC, Murphy T, Howard RS, et al:Rituximab using a thrice weekly dosing schedulein B-cell chronic lymphocytic leukemia andsmall lymphocytic lymphoma demonstratesclinical activity and acceptable toxicity. J ClinOncol 19:2153-2164, 2001.
34.
Dreger P, Montserrat E: Autologous andallogeneic stem cell transplantation forchronic lymphocytic leukemia. Leukemia16:985-992, 2002.
35.
Khouri IF, Keating M, Korbling M, et al:Transplant-lite: Induction of graft-vs-malignancyusing fludarabine-based nonablative chemotherapyand allogeneic blood progenitor-celltransplantation as treatment for lymphoid malignancies.J Clin Oncol 16:2817-2824, 1998.
36.
Aguilar-Santelises M, Rottenberg ME,Lewin N, et al: Bcl-2, Bax and p53 expression inB-CLL in relation to in vitro survival and clinicalprogression. Int J Cancer 69:114-119, 1996.
37.
Aviram A, Rabizadeh E, Zimra Y, et al:Expression of bcl-2 and bax in cells isolatedfrom B-chronic lymphocytic leukemia patientsat different stages of the disease. Eur JHaematol 64:80-84, 2000.
38.
Faderl S, Keating MJ, Do KA, et al: Expressionprofile of 11 proteins and their prognosticsignificance in patients with CLL.Leukemia 16:1045-1052, 2002.
39.
Klein U, Tu Y, Stolovitzky GA, et al:Gene expression profiling of B cell chroniclymphocytic leukemia reveals a homogeneousphenotype related to memory B cells. J ExpMed 194:1625-1638, 2001.
40.
Korz C, Pscherer A, Benner A, et al:Evidence for distinct pathomechanisms inB-cell chronic lymphocytic leukemia andmantle cell lymphoma by quantitative expressionanalysis of cell cycle and apoptosisassociatedgenes. Blood 99:4554-4561, 2002.
41.
Kitada S, Takayama S, De Riel K, et al:Reversal of chemoresistance of lymphoma cellsby antisense-mediated reduction of bcl-2 geneexpression. Antisense Res Dev 4:71-79, 1994.
42.
Pepper C, Thomas A, Hoy T, et al:Antisense-mediated suppression of Bcl-2 highlightsits pivotal role in failed apoptosis inB-cell chronic lymphocytic leukemia. Br JHaematol 107:611-615, 1999.
43.
Auer RL, Corbo M, Fegan CD, et al: Bcl-2 antisense (Genasense) induces apoptosis andpotentiates activity of both cytotoxic chemotherapyand rituximab in primary CLL cells (abstract3358). Blood 98(11 pt 1):808, 2001.
44.
Pahler JC, Ruiz S, Niemer I, et al: Effectsof the proteasome inhibitor, bortezomib,on apoptosis in isolated lymphocytes obtainedfrom patients with chronic lymphocytic leukemia.Clin Cancer Res 9:4570-4577, 2003.
45.
Cotter FE, Auer R, Corbo M, et al:Oblimersen sodium (G3139) sensitizes malignantB-cells to alemtuzumab (Ab) inducedapoptosis (abstract 910). Proc Am Soc ClinOncol 22:227, 2003.
46.
O’Brien S, Giles F, Rai K, et al: Bcl-2antisense (Genasense) as monotherapy forrefractory chronic lymphocytic leukemia(abstract 3213). Blood 98(11 pt 1):772, 2001.
47.
Decker T, Hipp S, Kreitman RJ, et al:Sensitization of B-cell chronic lymphocytic leukemiacells to recombinant immunotoxin byimmunostimulatory phosphorothioate oligodeoxynucleotides.Blood 99:1320-1326, 2002.
48.
Decker T, Schneller F, Sparwasser T, etal: Immunostimulatory CpG-oligonucleotidescause proliferation, cytokine production, and animmunogenic phenotype in chronic lymphocyticleukemia B cells. Blood 95:999-1006, 2000.
49.
Rai KR, O’Brien S, Cunningham C, etal: Genasense (Bcl-2 antisense) monotherapyin patients with relapsed or refractory chroniclymphocytic leukemia: Phase I and II results(abstract 1490). Blood 100(11 pt 1):384, 2002.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.