Biochemical Pharmacology of Pemetrexed
Pemetrexed (Alimta) is a novel antimetabolite that inhibits the folatedependentenzymes thymidylate synthase, dihydrofolate reductase, andglycinamide ribonucleotide formyltransferase. Pemetrexed has demonstratedactivity in clinical trials in a variety of tumor types, includinglung, breast, colon, mesothelioma, pancreatic, gastric, bladder, headand neck, and cervix. Pemetrexed is rapidly metabolized into activepolyglutamate forms that are potent inhibitors of several tetrahydrofolatecofactor-requiring enzymes critical to the synthesis of purines and thymidine.Functionally, pemetrexed acts as a prodrug for its polyglutamateforms. Two different transporters are known to take extracellular folates,and some antifolates, into the cell. These are the reduced folate carrierand the folate receptor. One of the many attributes that make pemetrexedunique is that methodology has been developed to eliminate and controlmany of its associated clinical toxicities. Multivariate analyses demonstratedthat pretreatment total plasma homocysteine levels significantlypredicted severe thrombocytopenia and neutropenia, with orwithout associated grade 3/4 diarrhea, mucositis, or infection. Routinevitamin B12 and folic acid supplementation have resulted in decreasedfrequency/severity of toxicities associated with pemetrexed without affectingefficacy, making this novel antifolate a safe and efficaciousanticancer agent.
ABSTRACT: Pemetrexed (Alimta) is a novel antimetabolite that inhibits the folatedependentenzymes thymidylate synthase, dihydrofolate reductase, andglycinamide ribonucleotide formyltransferase. Pemetrexed has demonstratedactivity in clinical trials in a variety of tumor types, includinglung, breast, colon, mesothelioma, pancreatic, gastric, bladder, headand neck, and cervix. Pemetrexed is rapidly metabolized into activepolyglutamate forms that are potent inhibitors of several tetrahydrofolatecofactor-requiring enzymes critical to the synthesis of purines and thymidine.Functionally, pemetrexed acts as a prodrug for its polyglutamateforms. Two different transporters are known to take extracellular folates,and some antifolates, into the cell. These are the reduced folate carrierand the folate receptor. One of the many attributes that make pemetrexedunique is that methodology has been developed to eliminate and controlmany of its associated clinical toxicities. Multivariate analyses demonstratedthat pretreatment total plasma homocysteine levels significantlypredicted severe thrombocytopenia and neutropenia, with orwithout associated grade 3/4 diarrhea, mucositis, or infection. Routinevitamin B12 and folic acid supplementation have resulted in decreasedfrequency/severity of toxicities associated with pemetrexed without affectingefficacy, making this novel antifolate a safe and efficaciousanticancer agent.
Antimetabolites are active chemotherapeuticagents in thetreatment of solid tumors andhematologic malignancies. The threemain categories of antimetabolites thatexist include folate antagonists (antifolates),purine analogs, and pyrimidineanalogs. Antifolates were firstused in the late 1940s with the discoveryof aminopterin, and soon after,methotrexate.[1] Since thesediscoveries, folate-dependent pathwayshave been an area of interest inthe development of new and effectiveanticancer agents.The antifolates interfere with thebinding of natural folate cofactors toimportant biosynthetic enzymes, suchas thymidylate synthase (TS), dihydrofolatereductase (DHFR), glycinamideribonucleotide formyl transferase(GARFT), and aminoimidazolecarboxamide formyl transferase(AICARFT). Inhibition of these enzymesresults in impeded synthesis ofnucleotides, which ultimately interfereswith DNA and RNA synthesis.[2] Resistance to antimetabolitescan occur by many mechanisms, includinguse of salvage pathways, geneamplification of the target enzymes,and reduced cell-membrane transportof the drug.Pemetrexed (Alimta) is approvedby the US Food and Drug Administration(FDA) for use in combinationwith cisplatin for the treatment ofmalignant pleural mesothelioma andsecond-line non-small-cell lung cancer.It is also advanced in clinical developmentin other tumor types. Itis an antifolate agent that inhibits atleast three key folate-requiring enzymesinvolved in DNA synthesis:TS, DHFR, and GARFT. Pemetrexedwas associated with sporadic toxicitiesin early clinical trials; however,methodology to eliminate and controlmany of these toxicities has been developed.This article will discuss thebiochemical pharmacology of pemetrexedaction and its modulation by physiological folates, the clinical significanceof the multiple mechanismsof action, and the impact of folic acidand vitamin B12 supplementation ondosing, toxicity, and efficacy of pemetrexed.The unique biochemical pharmacologyof pemetrexed probablycontributes to its clinical activity, andin particular, to its broad spectrum ofactivity in various tumor types.
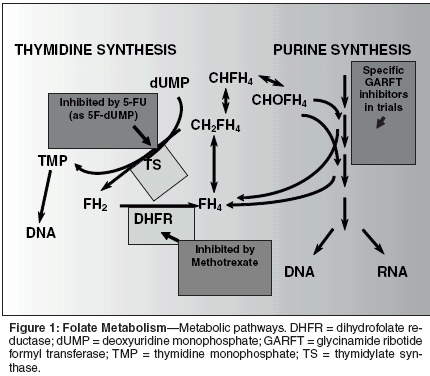
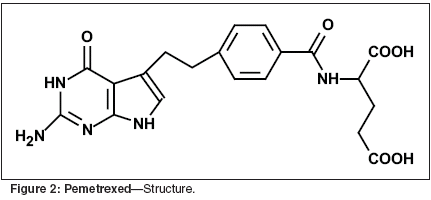
Function of Folic Acidin the CellFolic acid, in the form of 5,10-methylene-tetrahydrofolic acid, is essentialfor the synthesis of precursorsof purine nucleotides for DNA andRNA, and for the synthesis of thymidinenucleotide that is incorporatedexclusively into DNA. Folic acid, inthe fully reduced form, picks up onecarbonunits from amino acids andtransfers them into the synthetic pathwaysfor the purines. Also, whendeoxyuridine nucleotide is convertedto thymidine nucleotide by thethymidylate synthase enzyme, thefolate cofactor provides the carbonatom and is oxidized to dihydrofolateduring the reaction. The various antifolatesand the other commonly useddrugs that interact with folate pathwaysare illustrated in Figure 1. DHFRis inhibited by methotrexate, TS isinhibited by fluorouracil (5-FU), andDHFR, TS, and GARFT are inhibitedby pemetrexed.[2]PemetrexedPemetrexed is an analog of folicacid (Figure 2). Pemetrexed, as a singleagent and in combination regimens,has demonstrated activity inclinical trials in a range of solid tumors,including mesothelioma,[3]lung,[4-8] breast,[9,10] colon,[11-13]pancreatic,[14,15] gastric,[16] andbladder, head and neck, and cervix.[17] The toxicities reported in clinicalstudies of pemetrexed include dose-limiting myelosuppression, rash,mucosal toxicities, elevations in transaminases,and asthenia.Mechanism of ActionPemetrexed is a novel pyrrlol [2,3-d]pyrimidine-based antifolate antimetabolite,with multiple enzyme targetsthat are involved in both pyrimidineand purine syntheses. Pemetrexed wasoriginally investigated as a thymidylatesynthase inhibitor, but early dataindicated that two other enzymes,DHFR and GARFT, were also inhibitedby pemetrexed. Pemetrexed is rapidlymetabolized into activepolyglutamates derivatives. These arepotent inhibitors of several tetrahydrofolate(THF) cofactor-requiringenzymes critical to the synthesis ofpurines and thymidine nucleotides.Most antifolates and all naturalfolates are converted intracellularlyto polyglutamates by the enzyme folypolyglutamatesynthase (FPGS). Pemetrexedis one of the most avidsubstrates for FPGS.[18] Polyglutamatesof pemetrexed are morepotent inhibitors of the two target enzymes,TS and GARFT, than are themonoglutamate forms. Also polyglutamatesare retained in the celllonger due to their negative charges,thus increasing their potency due to amore prolonged inhibition of the targetenzymes. The addition of pemetrexedto cells in vitro leads to rapidbuildup of polyglutamates that resultin the suppression of TS. These polyglutamatesmay also inhibit GARFTand thus purine syntheses.[18]There are two features of pemetrexedpharmacology that may contributeto its selective antitumor effect.First, methylthiadenosine phosphorylase(MTAP) is a purine salvage pathwaythat is responsible for recyclingpurines within the cell. Cells that donot contain this pathway are more dependenton producing their own purinesthan those that utilize MTAP.MTAP is located on the 9P21 genomenext to the P16 putative tumorsuppressor gene. A mutation in the9P21 region that leads to a deletion ofP16 function is also likely to cause aloss of function of the adjacent MTAPgene, making the tumor cell more dependent on purine synthesis than itsnormal cellular counterpart. Becausepemetrexed polyglutamates inhibitGARFT and this de novo purine synthesis,cells with MTAP deletions maybe expected to be particularly sensitiveto pemetrexed. In patients withnon-small-cell lung cancer, approximately38% of tumors have been reportedto show MTAP deletion,regardless of histologic subtype,which may lead to enhanced action ofpemetrexed in those patients.The second feature that may contributeto the selective antitumor efficacyof pemetrexed relates to thespecific folic acid transporters thatmay target pemetrexed towards somemalignant cell types. For example, thefolate receptor has been shown to beoverexpressed in some tumors andalso to be capable of transporting pemetrexedinto the tumor cell. This isdicussed in more detail below.Pemetrexed thus possesses somecharacteristics that are more closelyrelated to targeted agents than traditionalagents, when the mechanism ofaction is examined in detail.PharmacokineticsEnd product reversal studies performedin cell culture indicate thatthe growth inhibition induced by lowconcentrations of pemetrexed may bereversed by the addition of thymidinealone, implying that the effective targetis TS. However, higher concentrations(> 0.1 μM) required inaddition a source of purine to overcomethe growth inhibition, implyingthat, at these higher concentrations,the inhibition of GARFT was also becomingsignificant.[19]Data derived from patients on numerousphase II trials of pemetrexeddemonstrated that the pharmacokineticsof pemetrexed behaved in a highlypredictable fashion with an initial/distributionhalf-life of 0.63 hours, andan effective terminal/elimination halflifeof 2.73 hours. The volume of distribution at steady state was 16.51 L.In particular, the plasma level exceeded0.1 μg/mL for 12 hours in all patientsand for 24 hours in many.Pemetrexed is not highly proteinboundin plasma so it appears that thelevels achieved clinically exceed by awide margin those necessary to causeinhibition of GARFT in cell culture.[20]Folate TransportThe efficacy of antifolates will beaffected by their transport into thecell. Two different transport systemsare known that transport folates andsome antifolates into cells. Followingtransport into the cell, conversion ofthe antifolate to its polyglutamate derivatesboth renders it more potentagainst the target enzymes and causeslonger retention in the cell. The hydrolysisof antifolate polyglutamatesthat can efflux through the cell membranemay also be an important featurein some cell types. In all of theseprocesses, there is the potential of naturalfolates to compete with antifolates,leading to the expectation thatthe nutritional status of the patientwith respect to folic acid may be animportant determinant of toxicity.One of the best known folate transportersis the reduced folate carrierthat has a high capacity and transportstetrahydrofolates, methotrexate,raltitrexed (Tomudex), and pemetrexed.Although the reduced folatecarrier has a high capacity, it has arelatively low affinity for most folatesand antifolates and its role in the transportof folic acid at physiological levelsis uncertain.
The second receptor is a highaffinityglycosylphosphatidylinositolanchoredreceptor for folate that worksby binding the molecules on the cellsurface and undergoing clustering toform structures called caveolae thatare internalized.[21] Although this receptor,which is known as the folatereceptor-alpha, has a high affinity, ithas a much lower capacity than thereduced folate carrier. Until recently,the folate receptor has not been consideredto be important for drug actionbecause of its low capacity;however, there is evidence to suggestthat it may be an important factor inthe action of some agents such as pemetrexedand CB 3717.In the case of mesothelioma inparticular, there is evidence that thefolate receptor-alpha, which potentiallytransports pemetrexed, is highlyoverexpressed.[22]SafetyThe safety profile is an importantfeature, as clinicians have reportedsafety problems and sporadic drugrelateddeaths with pemetrexed andsome other antifolates. One of themany unique characteristics of pemetrexedis the methodology that hasbeen developed to eliminate and/orcontrol its associated toxicities. Someof the problems with toxicity occurredwith pemetrexed in early studies thatwere conducted before the routine useof vitamin B12 and folic acid supplementationwas included in the studydesign.[10] Grade 4 neutropenia withgrade 3/4 infection, grade 3/4 diarrhea,or grade 3/4 mucositis were some potentially life-threatening toxicitiesseen in these early studies. Also, insome cases, lethal complications wereseen in these early studies, with a 4%incidence of drug-related deaths.[23]
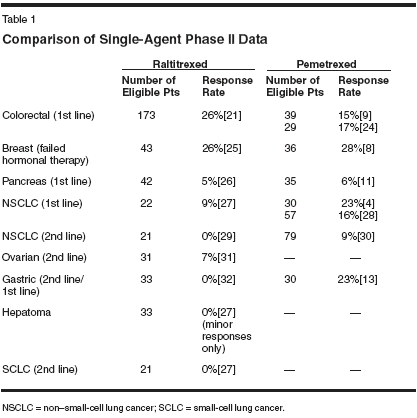
Folic Acid and Vitamin B12Because pemetrexed is an antifolate,one might expect the patient'sfolic acid status to predict toxicity.However, measurement of either plasmaor red cell folate did not provideinformation that allowed the patientswho developed toxicity to be identifiedin advance.[23,13,24,25] The reasoningthat folic acid levels are notvery predictive for antifolate toxicitiesis that plasma or red cells may beassociated with the fact that folic acidlevels actually do not reflect the functionalfolate status of the patient.(These are the folate pathways thatwere involved in generating the precursorsfor cell replication for DNAsynthesis.)The conversion of homocysteineto methionine is an important cellularprocess because methionine is involvedin a number of methylationreactions. The enzyme methioninesynthase is responsible for this conversionand used 5-methyltetrahydrofolateas a substrate. It is also a vitaminB12-dependent enzyme. If the patientis mildly vitamin B12- or folate-deficient,this enzyme will convert homocysteineto methionine moreslowly, resulting in an elevation ofthe plasma homocysteine level. Thus,the plasma homocysteine level is asensitive surrogate marker for thefolate status of the patient.Rationale for VitaminSupplementationIn the early phase II studies conductedprior to routine supplementationwith vitamin B12 and folic acid,the pretreatment homocysteine levelscorrelated very strongly with the neutropenia,thrombocytopenia, and diarrheareported with pemetrexedadministration. These data, coupledwith information based on experience with lometrexol (a GARFT inhibitor),led to the collection of several vitamin-deficiency markers from patientsaccrued on clinical trials during theearly phase II clinical development ofpemetrexed. Two multivariate analyseswere conducted that demonstratedthat pretreatment total plasmahomocysteine (tHcy) levels significantlypredicted severe thrombocytopeniaand neutropenia, with orwithout associated grade 3/4 diarrhea,mucositis, or infection. During thisperiod, a drug-related death rate of7% was observed early in a phase IIImesothelioma trial comparing pemetrexedplus cisplatin with cisplatinmonotherapy.A decision was made in December1999 that required all patients receivingpemetrexed be treated concomitantlywith folic acid and vitamin B12to minimize the risk of severe toxicity.The rationale for administering itto all patients rather than selectingpatients follows: although there is avery strong correlation of toxicity withelevated homocysteine levels that suggestthat it is indicative of poor folatestatus for the patient, there is not adefined level or cutoff point that allowsprecise identification of individualpatients. After this clinical practicewas introduced, the percentage of toxicdeaths decreased drastically. Thepercentage of hematologic grade 3 and4 toxicity decreased to approximately6%, grade 4 neutropenia to about 2%,and grade 4 thrombocytopeniadisappeared.The use of routine vitamin B12 andfolate supplementation with pemetrexedthus makes pemetrexed an extraordinarilysafe drug, withoutapparently interfering with its antitumoractivity.Clinical Activity of PemetrexedDoes pemetrexed work better invarious tumor types than other agents,including other antifolates? Based onclinical trial data, the only drug thatone may compare presently with pemetrexedis raltitrexed, a water-solublequinazoline antifolate that specificallyinhibits the TS enzyme. Table 1summarized response rates that havebeen reported for both drugs administered as single agents in clinical trials,albeit not by direct comparison in thestudy design. In a majority of the trialsshown in Table 1, investigatorsreported higher response rates withpemetrexed than with raltitrexed.ConclusionPemetrexed is a novel antifolatethat differs from related, licensed, andexperimental drugs both in cell membranetransport and intracellular loci.Pemetrexed, when administered withfolic acid and vitamin B12 supplementation,is safe, and has a very lowlevel of subjective and objective toxicity.Pemetrexed possesses substantialanticancer activity in a range ofsolid tumors, and may be given incombination with a number of majoranticancer agents.As approved by the FDA, pemetrexedin combination with cisplatinsignificantly prolonged survival in patientswith mesothelioma; explorationof other indications is ongoing and hasalso led to the approval of the drug insecond-line non-small-cell lung cancer.Vitamin B12 and folic acid supplementationdoes not adversely affect,and might improve, activity in stomachcancer and mesothelioma. Pemetrexeddemonstrated interesting activity inbreast cancer; however, there have beensome data suggesting that vitamin supplementationmay reduce the responserate in breast cancer patients. Furtherinvestigation in various tumor types iswarranted.
Disclosures:
The authors have nosignificant financial interest or other relationshipwith the manufacturers of any productsor providers of any service mentioned in thisarticle.
References:
1.
Calvert H: Folate status and the safetyprofile of antifolates. Semin Oncol 29(2 suppl5):3-7, 2002.
2.
Goldman ID, Zhao R: Molecular, biochemical,and cellular pharmacology ofpemetrexed. Semin Oncol 29(6 suppl 18):3-17,2002.
3.
Vogelzang NJ, Rusthoven JJ, SymanowskiJ, et al: Phase III study of pemetrexed in combinationwith cisplatin versus cisplatin alonein patients with malignant pleural mesothelioma.J Clin Oncol 21:2636-2644, 2003.
4.
Rusthoven JJ, Eisenhauer E, Butts C, etal: Multitargeted antifolate LY231514 as firstlinechemotherapy for patients with advancednon-small-cell lung cancer: A phase II study.J Clin Oncol 17:1194-1199, 1999.
5.
Shepherd FA, Dancey J, Arnold A, et al:Phase II study of pemetrexed disodium, amultitargeted antifolate, and cisplatin as firstlinetherapy in patients with advanced nonsmallcell lung carcinoma. Cancer 92:595-600,2001.
6.
Shepherd FA: Pemetrexed in the treatmentof non-small cell lung cancer. Semin Oncol 29(6suppl 18):43-48, 2002.
7.
AstraZeneca, data on file.8. Woll PJ, Basser R, Le Chevalier T, et al:Phase II trial of raltitrexed (‘Tomudex’) in advancedsmall-cell lung cancer. Br J Cancer76:264-265, 1997.
9.
O’Shaughnessy J: Pemetrexed: An activenew agent for breast cancer. Semin Oncol 29(6suppl 18):57-62, 2002.
10.
Miles DW, Smith IE, Coleman RE, etal: A phase II study of pemetrexed disodium(LY231514) in patients with locally recurrentor metastatic breast cancer. Eur J Cancer37:1366-1371, 2001.
11.
John W, Picus J, Blanke C, et al: Activityof multitargeted antifolate (pemetrexed disodium,LY231514) in patients with advancedcolorectal carcinoma. Cancer 88:1807-1813,2000.
12.
Hochster H: The role of pemetrexed inthe treatment of colorectal cancer. Semin Oncol29(6 suppl 18):54-56, 2002.
13.
Maughan TS, James RD, Kerr D, et al:Preliminary results of a multicenter randomizedtrial comparing 3 chemotherapy regimens(de Gramont, Likich and Ralitrexed) in metastaticcolorectal cancer. Proc Am Soc ClinOncol 18:A1007, 1999.
14.
Miller KD, Picus J, Blanke C, et al: PhaseII study of the multitargeted antifolateLY231514 (ALIMTA, MTA, pemetrexed disodium)in patients with advanced pancreaticcancer. Ann Oncol 11:101-103, 2000.
15.
Kindler H. Pemetrexed in pancreaticcancer. Semin Oncol 29(6 suppl 18):49-53,2002.
16.
Celio L, Buzzoni R, Longarini R, et al:Pemetrexed in gastric cancer: Clinical experienceand future perspectives. Semin Oncol 29(6suppl 18):63-68, 2002.
17.
Paz-Ares L, Ciruelos E, Garcia-Carbonero R, et al: Pemetrexed in bladder, headand neck, and cervical cancers. Semin Oncol29(6 suppl 18):69-75, 2002.
18.
Goldman ID, Zhao R: Molecular, biochemical,and cellular pharmacology ofpemetrexed. Semin Oncol 29(6 suppl 18):3-17,2002.
19.
Shih C, Chen VJ, Gossett LS, et al:LY231514, a pyrrolo[2,3-d]pyrimidine-basedantifolate that inhibits multiple folate-requiringenzymes. Cancer Res 57:1116-1123, 1997.
20.
Ouellet D, Periclou AP, Johnson RD, etal: Population pharmacokinetics of pemetrexeddisodium (ALIMTA) in patients with cancer.Cancer Chemother Pharmacol 46:227-234,2000.
21.
Smart EJ, Mineo C, Anderson RG, et al:Clustered folate receptors deliver 5-methyltetrahydrofolate to cytoplasm of MA104cells. J Cell Biol 134:1169-1177, 1996.
22.
Bueno R, Appasani K, Mercer H, et al:The α-folate receptor is highly activated inpleural mesothelioma. J Thorac CardiovascSurg 121:225-233, 2001.
23.
Niyikiza C, Hanauske AR, RusthovenJJ, et al: Pemetrexed safety and dosing strategy.Semin Oncol 29(suppl 18):24-29, 2002.
24.
Zalcberg JR, Cunningham D, VanCutsem E, et al: ZD1694: A novel thymidylatesynthase inhibitor with substantial activity inthe treatment of patients with advancedcolorectal cancer. Tomudex Colorectal StudyGroup. J Clin Oncol 14:716-721, 1996.
25.
Cunningham D, Zalcberg JR, Rath U, etal: Final results of a randomised trial comparingTomudex (raltitrexed) with 5-fluorouracilplus leucovorin in advanced colorectal cancer.Tomudex Colorectal Cancer Study Group. AnnOncol 7:407, 1996.
26.
Cripps C, Burnell M, Jolivet J, et al:Phase II study of first-line LY231514 (multitargetedantifolate) in patients with locally advancedor metastatic colorectal cancer: AnNCIC Clinical Trials Group Study. Ann Oncol10:1175-1179, 1999.
27.
Smith I, Jones A, Spielmann M, et al: Aphase II study in advanced breast cancer:ZD1694 (Tomudex) a novel direct and specificthymidylate synthase inhibitor. Br J Cancer74:479-481, 1996.
28.
Pazdur R, Meropol NJ, Casper ES, et al:Phase II trial of ZD1694 (Tomudex) in patientswith advanced pancreatic cancer. Invest NewDrugs 13:355-358, 1996.
29.
Clarke SJ, Abratt R, Goedhals L, et al:Phase II trial of pemetrexed disodium(ALIMTA, LY231514) in chemotherapy-naïvepatients with advanced non-small-cell lungcancer. Ann Oncol 13:737-741, 2002.
30.
Smit EF, Mattson K, von Pawel J, et al:ALIMTA (pemetrexed disodium) as secondlinetreatment of non-small-cell lung cancer:A phase II study. Ann Oncol 14:455-460, 2003.
31.
Gore ME, Earl HM, Cassidy J, et al: Aphase II study of Tomudex in relapsed epithelialovarian cancer. Ann Oncol 6:724-725, 1995.
32.
Meropol NJ, Pazdur R, Vincent M, et al:Phase II study of ZD1694 in patients with advancedgastric cancer. Am J Clin Oncol 19:628-630, 1996.