Pemetrexed in Pancreatic Cancer
Single-agent gemcitabine (Gemzar) is the standard of chemotherapyfor advanced pancreatic cancer, with no phase III trials to date havingshown significantly improved survival with gemcitabine-based combinationsvs single-agent treatment. The multitargeted antifolate agentpemetrexed (Alimta) shows synergistic effects in vitro in combinationwith gemcitabine, and activity and good tolerability when used as singleagenttreatment in advanced pancreatic cancer. In a phase II trial inpatients with advanced pancreatic cancer, the combination ofgemcitabine at 1,250 mg/m2 on days 1 and 8 plus pemetrexed at 500mg/m2 on day 8 after gemcitabine every 21 days resulted in a mediansurvival of 6.5 months and a 1-year survival rate of 29%. Neutropeniawas the primary toxicity, with grade 4 toxicity in 51% of patients. Thepromising results of this trial prompted the initiation of a phase IIItrial comparing gemcitabine at 1,000 mg/m2 on days 1, 8, and 15 every28 days vs the 21-day gemcitabine/pemetrexed regimen given with vitaminsupplementation in patients with pancreatic cancer. The primaryoutcome measure was overall survival, with secondary measures includingresponse rate, progression-free survival, and quality of life.While an increase in response and time to progression was reported forthe gemcitabine/pemetrexed combination, there were no significantdifferences in survival between treatment arms.
ABSTRACT: Single-agent gemcitabine (Gemzar) is the standard of chemotherapyfor advanced pancreatic cancer, with no phase III trials to date havingshown significantly improved survival with gemcitabine-based combinationsvs single-agent treatment. The multitargeted antifolate agentpemetrexed (Alimta) shows synergistic effects in vitro in combinationwith gemcitabine, and activity and good tolerability when used as singleagenttreatment in advanced pancreatic cancer. In a phase II trial inpatients with advanced pancreatic cancer, the combination ofgemcitabine at 1,250 mg/m2 on days 1 and 8 plus pemetrexed at 500mg/m2 on day 8 after gemcitabine every 21 days resulted in a mediansurvival of 6.5 months and a 1-year survival rate of 29%. Neutropeniawas the primary toxicity, with grade 4 toxicity in 51% of patients. Thepromising results of this trial prompted the initiation of a phase IIItrial comparing gemcitabine at 1,000 mg/m2 on days 1, 8, and 15 every28 days vs the 21-day gemcitabine/pemetrexed regimen given with vitaminsupplementation in patients with pancreatic cancer. The primaryoutcome measure was overall survival, with secondary measures includingresponse rate, progression-free survival, and quality of life.While an increase in response and time to progression was reported forthe gemcitabine/pemetrexed combination, there were no significantdifferences in survival between treatment arms.
The incidence of pancreatic cancerin France increased by approximately70% between 1980and 2000. According to European registrydata, only 4% of patients withpancreatic cancer survive for 5years.[1] Long-term survival is limitedto patients with resectable tumors,who account for only 10% to 20% ofpatients. The vast majority of patientspresent with locally advanced or metastaticdisease, creating a large populationof patients in need of palliativechemotherapy.Single-agent gemcitabine (Gemzar)is considered standard of care inchemotherapy for pancreatic cancer.The antimetabolite agent pemetrexed(Alimta) shows activity in pancreaticcancer and would appear to be a suitablecandidate for partnering withgemcitabine in this setting.Current Chemotherapyfor Pancreatic CancerFluorouracil (5-FU)-based regimenshad long been the mainstay ofchemotherapy for advanced pancreaticcancer. A phase III trial reportedby Burris et al in 1997 showed significantbenefits of gemcitabine vs 5-FUin this setting, with gemcitabine treatmentimproving 1-year survival (18%vs 2%).[2] A recent meta-analysis ofchemotherapy in pancreatic cancer hasconfirmed this superiority, with 5-FU-based chemotherapy shown to be superiorto best supportive care in termsof median overall survival, and single-agent gemcitabine shown to besuperior to 5-FU and other chemotherapeuticagents.[3]A current issue in identifying optimalchemotherapy in this setting iswhether gemcitabine-based combinationsare superior to gemcitabine as asingle agent. A number of phase IItrials of gemcitabine in combinationwith other agents (eg, cisplatin, 5-FU,docetaxel [Taxotere], oxaliplatin [Eloxatin],and irinotecan [Camptosar]) haveyielded response rates of 20% to 30%and have reported 1-year survival ratesof up to 30%.
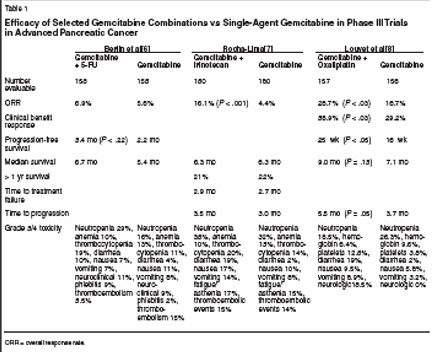
However, to date, no phase III trialhas shown superiority of combinationtreatment over single-agent gemcitabinein terms of overall survival. Forexample, increased progression-freesurvival was found in trials of gemcitabine/cisplatin vs gemcitabine reportedby Colucci et al[4] (5 vs 2 months, P= .048) and Heinemann et al[5] (4.6 vs2.5 months, P = .016), with no differencein overall survival found in eitherstudy (7 vs 4.7 months and 7.6 vs 6.0months, respectively).Berlin et al[6] reported greater progression-free survival in a phase IIItrial (Eastern Cooperative OncologyGroup [ECOG] EE2297) with gemcitabineplus 5-FU (3.4 vs 2.2 months,P = .022) with no difference in overallsurvival (6.7 vs 5.4 months);Rocha-Lima et al[7] reported no differencein time to progression (3.5 vs3.0 months) or overall survival (6.3vs 6.6 months) with gemcitabine/irinotecanvs gemcitabine. A trial of gemcitabine/oxaliplatin (GEMOX) vsgemcitabine reported by Louvet etal[8] showed that GEMOX achievedsignificantly better results than gemcitabinein terms of response rate, clinicalbenefit, and progression-freesurvival, but the difference did notreach the significant level for overallsurvival. These phase III studies aresummarized in Table 1.In addition, biological agents (marimastat,BAY 12-9566, tipifarnib[Zarnestra]) have been combined withgemcitabine in four phase III trialswith no improvement in outcomecompared with gemcitabine alone beingobserved.[9]Rationale for Combination ofPemetrexed and GemcitabinePemetrexed is an antimetabolitethat targets several enzymes in thepyrimidine and purine synthesis pathways,including thymidylate synthase,dihydrofolate reductase, and glycinamideribonucleotide formyltransferase.It has shown clinical activityin multiple tumor types, including pancreascancer, and in treatment-refractory/resistant tumors.The dose-limiting toxicity of pemetrexedis myelosuppression, with othertoxicities including fatigue, rash,mucositis, and diarrhea. Folic acid andvitamin B12 supplementation is nowroutinely given in clinical trials to reducethe risk of severe toxicity. Thedrug has a convenient administrationschedule consisting of a 10-minuteintravenous (IV) infusion every 3weeks.In a phase II trial, Miller et al administeredpemetrexed at a dose of600 mg/m2 every 3 weeks withoutvitamin supplementation as first-linetreatment to 42 chemotherapy-naivepatients with advanced pancreatic cancer.[10] The patients had a medianage of 60 years, and 79% had stageIV disease.The objective response rate was5.7%, with one patient having a completeresponse lasting for 16.2 monthsand one having a partial response lasting6.9 months. The stable diseaserate was 40%; five patients with stabledisease were alive at the time ofanalysis, with survival durations of6+ to 19+ months. Median survivalwas 6.5 months, and the 1-year survivalrate was 28%.Treatment was well tolerated.Grade 3/4 hematologic toxicities includedneutropenia in 17%/24% ofpatients, leukopenia in 31%/12%,thrombocytopenia in 10%/7%, andanemia in 14%/5%. Grade 3 aspartateaminotransferase and alanine aminotransferaseelevations occurred in15% and 7.5% of patients, respectively,grade 3 nausea occurred in17%, and grade 3/4 vomiting occurredin 2%/5%.The demonstration of pemetrexed'sactivity in advanced pancreas cancerprompted exploration of the potentialfor combining pemetrexed and gemcitabinein this setting. In addition tothe single-agent activity of both, therationale for combination includes thefact that the two agents are antimetabolitesthat act via different mechanismsof action. The combinationshows synergistic effects in vitro, withcytotoxic effects greatest when gemcitabineexposure occurs before pem-etrexed exposure (suggesting thatgemcitabine inhibition of thymidylatesynthase could enhance inhibition ofthis enzyme by pemetrexed). Bothagents have favorable side-effect profileswhen used as monotherapy, suggestingthe potential for use of fulldoses of each in combination.
Adjei et al reported from theirphase I study in patients with solidtumors that the recommended dose ofthe combination was gemcitabine at1,250 mg/m2 on days 1 and 8 andpemetrexed at 500 mg/m2 on day 8every 21 days.[11] The combinationproduced promising responses in thephase I trial, with 12 partial responsesobserved in 35 evaluable patients. Thecombination was subsequently evaluatedin a phase II trial in pancreaticcancer.
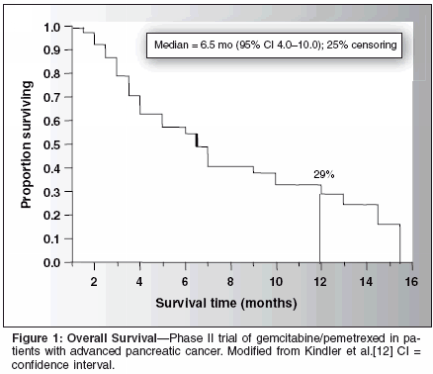
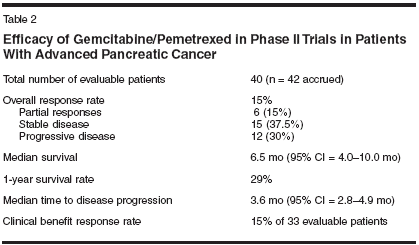
Phase II Trial of Gemcitabine/Pemetrexed in PancreaticCancerIn an open-label phase II trial ofKindler et al, patients with histologicallyor cytologically confirmed stageIII or IV adenocarcinoma of the pancreasnot amenable to curative resectionwere treated with gemcitabine at1,250 mg/m2 by 30-minute infusionon days 1 and 8 plus pemetrexed at500 mg/m2 by 10-minute infusion onday 8 at 90 minutes after gemcitabineevery 21 days.[12,13]To be eligible for inclusion in thestudy, patients had to have no priorsystemic chemotherapy; prior use of5-FU as a radiosensitizer was allowedif treatment occurred > 4 weeks priorto enrollment, and prior radiation to< 25% of bone marrow was permittedif treatment was completed > 4 weeksprior to enrollment. All patients receiveddexamethasone pretreatment toprevent skin rash. Routine vitamin B12supplementation was initiated after thestudy had been under way for 2months. The study utilized a two-stagesequential design. In the first part ofthe study, 21 patients were enrolled;if fewer than two patients had tumorresponse, the study was to be stopped.An additional 20 patients were to beenrolled in the second part of the trial;if fewer than five patients overall hada tumor response, the regimen was tobe deemed unsuitable for additionalinvestigation.A total of 42 patients (64% male)with a median age of 60 years (range:34-79 years) were enrolled in thestudy. The majority of patients (95%)had stage IV disease, and most patientshad Karnofsky performancescores of 80 (36%) or 90 (55%). Intotal, 12% of patients had prior adjuvanttherapy (5-FU in all cases). Atotal of 212 treatment cycles were givenin the study, with a range of 1 to17; five patients received 10 or morecycles.Results are shown in Tables 2 and3. The objective response rate was15%, with six partial responses beingobserved. Median time to disease progressionwas 3.6 months. Median survivalwas 6.5 months (Figure 1), andthe 1-year survival rate was 29%. Neutropeniawas the primary hematologictoxicity, with grade 3/4 toxicityoccurring in 22%/51% of patients. Severenonhematologic toxicity was infrequent.Gemcitabine/Pemetrexed:Phase III TrialThe promising findings in thisstudy, particularly with regard to survival,prompted Richards et al[14] toconduct a phase III trial (N = 585)examining the gemcitabine/pemetrexedcombination. In this internationaltrial encompassing 19 countries,patients with locally advanced or metastaticadenocarcinoma of the pancreaswith bidimensionallymeasurable disease who have receivedno prior chemotherapy have been randomizedto receive either (1) gemcitabineat 1,000 mg/m2 on days 1, 8,and 15 every 28 days or (2) gemcitabineat 1,250 mg/m2 on days 1 and 8and pemetrexed at 500 mg/m2 on day8 after gemcitabine administrationevery 21 days. Investigators stratifiedpatients according to (1) ECOG performancestatus (0/1 vs 2), (2) stageII/III vs IV, (3) center, and (4) homocysteine.According to standardclinical practice, patients in the combinationarm received folic acid andvitamin B12 supplementation, as wellas dexamethasone (to prevent skinrash). The primary outcome measurewas overall survival; secondary outcomemeasures consisted of progression-free survival, response rate,quality of life, and toxicity.Median overall, 1-year, and progression-free survival did not differsignificantly between the gemcitabine/pemetrexed and gemcitabinearms (6.2 vs 6.3 months, 21.4% vs20.1%, and 3.9 vs 3.3 months, respectively).The intent-to-treat overallresponse was significantlyincreased in the gemcitabine/pemetrexedarm (14.8% vs 7.1%; P = .004),as was median time to progression(5.2 vs 3.6 months; P = .042).Overall quality of life (assessed with the European Organisation forResearch and Treatment of CancerQuality of Life Questionnaire Core30 [EORTC QLQ-C30]) was well preservedin both arms. Significantlygreater grade 3/4 toxicity was seen inthe gemcitabine/pemetrexed arm: neutropenia(45.1% vs 12.8%), thrombocytopenia(17.9% vs 6.2%), anemia(13.9% vs 2.9%), febrile neutropenia(9.9% vs 0.4%) (all P < .001), andfatigue (15% vs 6.6%; P < .05). Otherwise,both regimens were well toleratedby study patients. The authorsconcluded that gemcitabine monotherapyremained the standard for first-linetherapy in patients with locally advancedor metastatic pancreatic cancer.ConclusionPancreatic cancer remains a challengefor the oncologist. Since theapproval of gemcitabine for treatmentof pancreatic cancer, no phase III trialhas shown an improvement in overallsurvival with gemcitabine-based combinationtherapy over gemcitabine single-agent therapy in this setting. Whilethe combination of pemetrexed andgemcitabine demonstrated promisingactivity in a phase II trial in patientswith advanced pancreatic cancer, aphase III trial comparing gemcitabine/pemetrexed with gemcitabinealone did not report significant differencesin survival. In this trial, the combinationof pemetrexed/gemcitabinedid improve overall response and timeto progression. Further investigationmay be required to determine whetherthe addition of novel agents to thecurrent standard of gemcitabine mightimprove survival and quality of life inadvanced pancreatic cancer.
Disclosures:
The authors have nosignificant financial interest or other relationshipwith the manufacturers of any productsor providers of any service mentioned in thisarticle.
References:
1.
Faivre J, Forman D, Esteve J, et al: Survivalof patients with primary liver cancer, pancreaticcancer and biliary tract cancer in Europe.Eur J Cancer 34:2184-2190, 1998.
2.
Burris HA III, Moore MJ, Andersen J, etal: Improvements in survival and clinical benefitwith gemcitabine as first-line therapy forpatients with advanced pancreas cancer: A randomizedtrial. J Clin Oncol 15:2403-2413,1997.
3.
Fung MC, Ishiguro H, Takayama S, et al:Survival benefit of chemotherapy treatment inadvanced pancreatic cancer: A meta-analysis(abstract 1155). Proc Am Soc Clin Oncol22:288, 2003.
4.
Colucci G, Giuliani F, Gebbia V, et al:Gemcitabine alone or with cisplatin for thetreatment of patients with locally advanced and/or metastatic pancreatic carcinoma: A prospective,randomized phase III study of the GruppoOncologia dell’Italia Meridionale. Cancer94:902-910, 2002.
5.
Heinemann V, Quietzsch D, Gieseler F, etal: A phase III trial comparing gemcitabine pluscisplatin vs. gemcitabine alone in advancedpancreatic carcinoma (abstract 1003). Proc AmSoc Clin Oncol 22:250, 2003.
6.
Berlin JD, Catalano P, Thomas JP, et al:Phase III study of gemcitabine in combinationwith fluorouracil versus gemcitabinealone in patients with advanced pancreaticcarcinoma: Eastern Cooperative OncologyGroup Trial E2297. J Clin Oncol 20:3270-3275, 2002.
7.
Rocha-Lima CMS, Rotche R, Jeffery M,et al: A randomized phase 3 study comparingefficacy and safety of gemcitabine (GEM) andirinotecan (I), to GEM alone in patients (pts)with locally advanced or metastatic pancreaticcancer who have not received prior systemictherapy (abstract 1005). Proc Am Soc ClinOncol 22:251, 2003.
8.
Louvet C, Labianca R, Hammel P, etal: Gemcitabine versus GEMOX(gemcitabine + oxaliplatin) in nonresectablepancreatic adenocarcinoma: Final results ofthe GERCOR/GISCAD Intergroup phase III(abstract 4008). Proc Am Soc Clin Oncol23:LBA, 2004.
9.
Heinemann V: Gemcitabine-based combinationtreatment of pancreatic cancer. SeminOncol 29(suppl 3):25-35, 2002.
10.
Miller KD, Picus J, Blanke C, et al: PhaseII study of the multitargeted antifolateLY231514 (ALIMTA, MTA, pemetrexed disodium)in patients with advanced pancreaticcancer. Ann Oncol 11:101-103, 2000.
11.
Adjei AA, Erlichman C, Sloan JA, et al:Phase I and pharmacologic study of sequencesof gemcitabine and the multitargeted antifolateagent pemetrexed in patients with advancedsolid tumors. J Clin Oncol 18:1748-1757, 2000.
12.
Kindler HL, Dugan W, Hochster H, etal: Clinical outcome in patients (pts) with advancedpancreatic cancer treated withpemetrexed/gemcitabine (abstract 499). ProcAm Soc Clin Oncol 21:5, 2002.
13.
Kindler HL: The pemetrexed/gemcitabine combination in pancreatic cancer.Cancer 95(suppl):928-932, 2002.
14.
Richards DA, Kindler HL, Oettle H, etal: A randomized phase III study comparinggemcitabine + pemetrexed versus gemcitabinein patients with locally advanced and metastaticpancreas cancer (abstract 4007). Proc Am SocClin Oncol 23:LBA, 2004.