Pemetrexed in Gastric Cancer
Gastric cancer is a major clinical challenge, with poor overall prognosisand limited life expectancy for patients with advanced disease.Even with surgery and other modalities, palliation is often difficult.Improvement of response rates has evolved with the development ofstandard regimens and those incorporating newer chemotherapy agents,such as oral fluoropyrimidines, the taxanes, camptothecins, novel platinums(eg, oxaliplatin [Eloxatin]), and antifolates (eg, pemetrexed[Alimta]). Ongoing trials with these regimens aim toward improvingsurvival, as well as improving the safety profile. It is hoped that in conjunctionwith molecular research in the pathogenesis of gastric cancerand development of targeted therapies in this disease, these trial datamight lead to the evolution of treatment strategies that could prove effective.
ABSTRACT: Gastric cancer is a major clinical challenge, with poor overall prognosisand limited life expectancy for patients with advanced disease.Even with surgery and other modalities, palliation is often difficult.Improvement of response rates has evolved with the development ofstandard regimens and those incorporating newer chemotherapy agents,such as oral fluoropyrimidines, the taxanes, camptothecins, novel platinums(eg, oxaliplatin [Eloxatin]), and antifolates (eg, pemetrexed[Alimta]). Ongoing trials with these regimens aim toward improvingsurvival, as well as improving the safety profile. It is hoped that in conjunctionwith molecular research in the pathogenesis of gastric cancerand development of targeted therapies in this disease, these trial datamight lead to the evolution of treatment strategies that could prove effective.
ABSTRACT: Gastric cancer is a major clinical challenge, with poor overall prognosis and limited life expectancy for patients with advanced disease. Even with surgery and other modalities, palliation is often difficult. Improvement of response rates has evolved with the development of standard regimens and those incorporating newer chemotherapy agents, such as oral fluoropyrimidines, the taxanes, camptothecins, novel platinums (eg, oxaliplatin [Eloxatin]), and antifolates (eg, pemetrexed [Alimta]). Ongoing trials with these regimens aim toward improving survival, as well as improving the safety profile. It is hoped that in conjunction with molecular research in the pathogenesis of gastric cancer and development of targeted therapies in this disease, these trial data might lead to the evolution of treatment strategies that could prove effective.
Gastric cancer continues to be a major challenge to clinical oncologists because of its poor overall prognosis. In addition, the life span of patients with advanced disease is very limited, and their quality of life often leaves much to be desired. Palliation is often difficult, even when employing aggressive modalities such as surgery.
In 2001, Macdonald and colleagues of the US Intergroup study 116 apparently revolutionized this field with a randomized trial (n = 556 with resected adenocarcinoma of the stomach or gastroesophageal junction) showing that combined chemoradiation reduced the risk of recurrence and death by almost 40% [1]. Nevertheless, some have critiqued these findings, and it was realized that the combined treatment regimen was associated with the risk of severe toxicity- difficult for a large proportion of these patients who are fragile after surgery.
There is a strong need for better treatment strategies, but targeted therapies that have reported limited but objective success in breast, colon, renal, and lung cancer, as well as the rare gastrointestinal stromal tumors (GISTs), have thus far failed to achieve significant activity. Along with the development of innovative treatment modalities, classical chemotherapeutic agents continue to be investigated in this disease. Thus, advances in more conventional therapy approaches are the focus of this article.
Gastric Cancer: General Outlook
Figure 1 illustrates the generalized overall prognosis of gastric cancer. Out of 100 consecutive, unselected patients who present clinically (and undergo medical and/or surgical intervention), approximately 15 are cured. This figure derives from the generalized clinical observation that 30 patients will present with uncurable stage IV disease; 20 will have unresectable tumors (either due to their locally advanced nature or associated comorbid conditions). Of the 50 who will undergo surgery, 5 will generally die from postoperative complications, and 10 will have positive surgical margins, which lack curative treatment. The standard recommendations of management for each condition are also illustrated in Table 1.
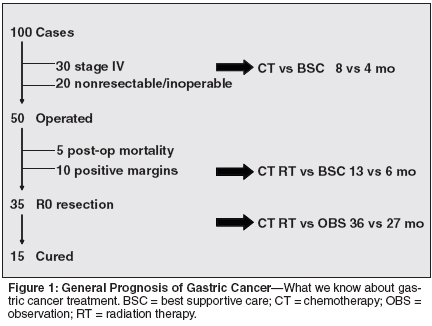
Stage IV patients benefit from chemotherapy either at presentation or relapse; in fact, there are three randomized trials comparing chemotherapy vs best supportive care (BSC) that demonstrate doubling of median survival. Patients who undergo an R1 or R2 resection (for microscopically and macroscopically positive margins, respectively) appear to benefit substantially from combined-modality chemoradiation therapy, as demonstrated by a randomized trial of Moertel et al that accrued 62 patients with resectable but poor-prognosis gastric carcinoma.[2]

Finally, patients who undergo an R0 resection (complete) benefit from adjuvant, combined chemoradiation therapy as well.[1] Thus, aggressive medical intervention with this combined- modality approach impacts the natural history of the disease, regardless of disease stage. Despite these therapeutic advances, what are termed the "absolute figures" are dismal (ie, percent of cure rates and median survival times of the various disease stages) so that, although the absolute benefit of treatment expressed as percent of improvement vs no treatment is high, the absolute number itself remains very small.
Evolution of Chemotherapy for Advanced-Stage Patients
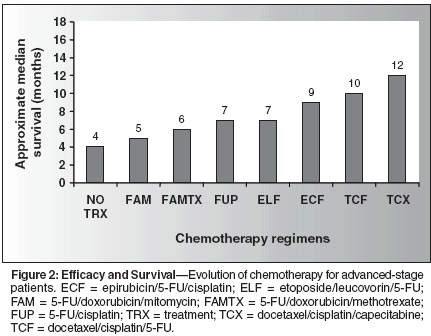
The clinical research and drug development during the past 20 years have produced a series of incremental improvements in the median survival of these patients. In general, one may state that progress has been achieved even in the most prohibitive disease settings. Figure 2 illustrates this concept. From the median survival of 3 to 4 months reported with no treatment, the combinations of fluorouracil (5-FU)/doxorubicin (Adriamycin)/mitomycin (FAM),[3] 5-FU/doxorubicin,[4] 5-FU/doxorubicin/ methotrexate (FAMTX),[5,6] 5-FU/cisplatin (Platinol) (FUP),[6] and 5-FU/etoposide/leucovorin (ELF)[7,8] progressively increased median survival rates. Comparable improvements were obtained with epirubicin/cisplatin/5-FU (ECF) with increases in survival ranging from 8.7 to 8.9 months, with improved safety profile and quality of life.[9,10] Finally, the recent use of the taxane docetaxel (Taxotere) in combination with cisplatin and 5-FU (TCF) produced one of the greatest durations of median survival reported in randomized trials in advanced gastric cancer.[11,12]
In a large international ongoing (n = 232; target accrual of 460) phase III randomized trial (V325) in advanced gastric cancer, Ajani et al reported significantly greater survival duration of 10.2 months (P = .064), with 38.7% response vs the standard FUP that achieved 8.5 months, with a significantly lower response of 23.2% (P = .012).[11] Interestingly, substitution of the oral fluoropyrimidine capecitabine for 5-FU resulted in a median survival of 11.9 months, with a 53% response rate, as reported recently by Kang et al who employed a docetaxel/ capecitabine (Xeloda)/cisplatin combination in first-line therapy in a phase I/II trial (n = 35).[12] Despite the trend of progressive improvements illustrated by Figure 2, the overall number remains small. This is in part why no worldwide consen-sus exists for a standard chemotherapy regimen in gastric cancer. In general, ECF is considered standard therapy in the United Kingdom. Cisplatin/ epirubicin/leucovorin/5-FU (PELF) is also common in Italy.[13] FUP is a standard therapy in the United States and France, whereas clinicians in Germany use both FUP and ELF, as espoused by Vanhoefer et al in their phase III trial (n = 399; median survial = 7.2 months).[14]
A relevant piece of information that is generally not available in reports published in the literature is the percentage of patients with advanced disease who do not receive chemotherapy. This may be due to a lack of ability to tolerate aggressive therapy based on low performance status, deteriorating medical characteristics, or a referral pattern that leads them away from specialized treatment centers. It is likely, though, that this percentage of untreated patients is substantial.
Contradictions of Chemotherapy for Advanced Gastric Cancer
An observation gleaned from Figure 2 is the relatively large number of agents (and combinations) that possess objective activity in gastric cancer. Notably, as with other gastrointestinal neoplasms, standard therapy is dominated by 5-FU. Even more intriguing is the response rate in clinical trials of gastric cancer. For untreated patients, the response rates ranged between 25% and 60%. Thus, one would conclude on the basis of response alone that there are at least eight active chemotherapeutic agents in this disease. However, this parameter alone may not reflect major clinical impact by this avenue of medical intervention. Hence, this contradiction typifies a clinical aspect of gastric cancer (also seen in other malignancies). Several active agents exist and achieve relatively high response rates, but these are generally of short duration. Moreover, a very short interval from disease progression to death occurs, with a relative short overall survival duration or percentage- 60% to 80% of the patients in randomized trials die from this disease within 12 months.
Activity of New Chemotherapeutic Agents
The development of oral fluoropyrimidines, the taxanes, camptothecins, platinum analogs, and antifolates have been major avenues of clinical research thus far. Generally, the goal of the first class of agents is to simplify the treatment, and thus, no increased efficacy may reasonably be expected; rather, convenience and a more favorable toxicity profile than the standard. Although the taxanes and camptothecins are new entries into the armametarium for treatment of gastric cancer, given their high rates of toxicity, they either will add substantial efficacy or the therapeutic margin will limit their clinical use.
The novel platinum analog oxaliplatin and the antifolates possess a favorable toxicity profile, but similarity with their parental compounds might only allow for similar efficacy. Thus, it appears that the overall benefit from the above approaches utilizing conventional chemotherapy may be limited, and a plateau in terms of efficacy may be near.
Oral Fluoropyrimidines
A combination of the fluorouracil prodrug tegafur and the dihydropyrimidine dehydrogenase inhibitor uracil (UFT), capecitabine, and S1 (a dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine) have all been investigated in several phase II studies of advanced gastric cancer, yielding an approximate response rate of 30%.[15,16] Three trials that each accrued between 36 and 45 patients employed single-agent UFT. Similarly, two small studies (n = 32 and n = 35, respectively) were conducted with capecitabine alone or in combination with docetaxel and cisplatin. Capecitabine alone had a response of 19.4%, with median survival of 247.5 days.[12,17]
S1, the compound that contains both a dihydropyrimidine dehydrogenase inhibitor and an orotate phosphoribosyl transferase inhibitor, is available in Japan, and four studies employed it as a single agent in this disease. In summary, one may say that the oral fluoropyrimidines achieve marginal activity when employed as single agents.
The Taxanes
In an MTT assay of 88 gastric cancer specimens, Maeda et al reported that docetaxel and paclitaxel showed a higher efficacy rate vs mitomycin, 5-FU, and cisplatin, with the patterns of antitumor activity of the taxanes independent from those of the conventional agents.[18] Nine singleagent phase II studies have been reported with the taxanes: five employing docetaxel and four using paclitaxel. The response rates reported in these studies are very similar, ranging between 5% and 40%. The two taxanes are considered equivalent, and perhaps might be used in patients resistant to FUP.
Camptothecins and Oxaliplatin
Few single phase II single-agent trials of irinotecan have been published, and investigators have reported response rates ranging between 0% and 30%.[19-21] For example, Kohne et al reported a response rate of 20%, with a median survival of 7.1 months in a study of 35 patients with ad advanced gastric cancer.[21] A new camptothecin analog rubitecan has also been studied as a single agent, but no activity was reported. Thus, although this class of compound may be potentially useful as a combination partner, their use as single agents do not appear beneficial.
There are no studies of single-agent oxaliplatin yet published in advanced gastric cancer. However, the ongoing REAL-2 phase II/III randomized, multicenter trial in the United Kingdom is investigating oxaliplatin in a 22 design by substituting either protracted venous infusion 5-FU with capecitabine or cisplatin with oxaliplatin.[ 22] Preliminary efficacy analyses indicated that the epirubicin/ oxaliplatin/capecitabine (EOX) regimen achieved an improved response rate of 52% vs epirubicin/cisplatin/ 5-FU (ECF, 31%), epirubicin/oxaliplatin/ 5-FU (EOF, 33%), or epirubicin/ cisplatin/capecitabine (ECX, 35%).
The preliminary toxicity results on the first 204 randomized patients accrued suggested that oxaliplatin-containing combinations are associated with milder toxicity vs cisplatin-containing regimens. The dose-limiting toxicities associated with the fluoropyrimidines were stomatitis, palmar plantar erythema, and diarrhea, with 5.1% of capecitabine-treated patients reporting grade 3/4 toxicities. Moreover, nonhematologic grade 3/4 toxicity affected 10.7% of patients who received capecitabine at 1,250 mg/m2 (the second dose escalation), confirming this optimal dose-one that will continue up to the target accrual of 600 patients. The primary end point of this trial is survival, defined as time from randomization to death (from any cause).
Antifolates and Pemetrexed
Methotrexate is an active compound against gastric cancer. It appears that its role as a component in the FAMTX combination serves not only to modulate 5-FU, but itself to act as a cytotoxic agent.
Pemetrexed is a novel antifolate (ie, it inhibits at least three folatedependent enzymes) that is approved with cisplatin by the US Food and Drug Administration for the treatment of mesothelioma. It has also been widely investigated in clinical trials either as a single agent or in combination for treatment of non-small-cell lung (NSCLC), breast, pancreatic, colon, and gastric cancers.
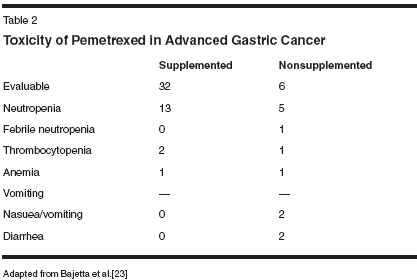
There is one recently published trial of pemetrexed in advanced gastric cancer.[23] Bajetta et al accrued 38 previously untreated patients with stage IV disease (39% had three or more metastatic sites), and administered pemetrexed 500 mg/m2 as a 10- minute infusion every 21 days. While the original study design did not incorporate folate or vitamin B12 supplementation, toxicity was prominent in the first six patients. Subsequently, the trial was amended to include supplementation the week before treatment with pemetrexed (folic acid given at 5 mg/d on days -2 to +2 of every cycle). Folic acid and vitamin B12 supplementation are now included in clinical applications of pemetrexed, either in the clinical or trial setting.
The efficacy and safety profiles for this trial are summarized in Tables 1 and 2. Toxicity was very mild in the cohort of patients receiving vitamin supplementation. In particular, the "subjective" adverse events (ie, mucositis, diarrhea, and vomiting) were mild. The intent-to-treat response rate was 21% in previously untreated patients (6 nonsupplemented patients and 30 supplemented), including two complete and six partial responses. (The analysis incorporated data from the earlier cohort who did not receive vitamin supplementation, and in whom investigators noted a lack of objective response.) The overall median survival was 7.8 months, and duration of response was 4.6 months. The authors noted that the promising activity of pemetrexed warranted combination studies.
This reported level of activity with pemetrexed thus compares well with the activity of the other chemotherapeutic agents described in this article. Notably, this activity has been obtained with very mild toxicity (provided vitamin supplementation is given). Therefore, pemetrexed may be a good candidate for inclusion into combination chemotherapy regimens in gastric cancer. Moreover, the hypothesis that the multitargeted antifolates synergize in vitro with a host of active compounds in gastric cancer (ie, cisplatin, epirubicin, oxaliplatin) lend further support to a role for pemetrexed in treatment of gastric cancer.
Conclusions
The treatment of advanced gastric cancer remains largely unsatisfactory; despite the development of several active agents, median survival remains below 10 months at best. Incremental improvements may be obtained by the substitution of key drugs in standard combinations (FUP or ECF). The oral fluoropyrimidines (ie, UFT, capecitabine, S1) might substitute for 5-FU when issues of administration convenience are paramount; accordingly, pemetrexed might also be used when both enhanced efficacy and convenience are important.
The results of the REAL-2 trial might validate the concept of oxaliplatin replacing cisplatin to reduce toxicity while maintaining efficacy.[24] The added value of the taxanes also needs to be confirmed due to the added toxicity of the triple-agent TCF combination. Finally, these trials, in conjunction with ongoing research in the molecular pathogenesis of gastric cancer, may yet offer key hints for the development of targeted therapies that could prove effective in this disease.
Disclosures:
Dr. Sobrero has acted as a consultant for Eli Lilly, Sanofi Aventis, and Roche.
References:
1. Macdonald JS, Smalley SR, Benedetti J, et al: Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725-730, 2001.
2. Moertel CG, Childs DS, O’Fallon JR, et al: Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol 2(11):1249-1254, 1984.
3. Coombes RC, Schein PS, Chilvers CE, et al: A randomized trial comparing adjuvant fluorouracil, doxorubicin, and mitomycin with no treatment in operable gastric cancer. International Collaborative Cancer Group. J Clin Oncol 8:1362-1369, 1990.
4. Krook JE, O’Connell MJ, Wieand HS, et al: A prospective, randomized evaluation of intensive-course 5-fluorouracil plus doxorubicin as surgical adjuvant chemotherapy for resected gastric cancers. Cancer 67:2454-2458, 1991.
5. Klein HO: Long-term results with FAMTX (5-fluorouracil, adriamycin, methotrexate) in advanced gastric cancer. Anticancer Res 9:1025-1026, 1989.
6. Kim NK, Park YS, Heo DS, et al: A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouarcil alone in the treatment of advanced gastric cancer. Cancer 71:3813-3818, 1993.
7. Wilke H, Preusser P, Fink U, et al: High dose folinic acid/etoposide/5-fluorouracil in advanced gastric cancer-A phase II study in elderly patients or patients with cardiac risk. Invest New Drugs 8:65-70, 1990.
8. Taal BG, Teller FG, ten Bokkel Huinink WW, et al: Etoposide, leucovorin, 5-fluorouracil (ELF) combination chemotherapy for advanced gastric cancer: Experience with two treatment schedules incorporating intravenous and oral etoposide. Ann Oncol 5:90-92, 1994.
9. Waters JS, Norman A, Cunningham D, et al: Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: Results of a randomised trial. Br J Cancer 80:269-272, 1999.
10. Webb A, Cunningam D, Scarffe JH: Randomized trial comparing epirubicin, cisplatin and fluorouracil versus fluorouracil, doxorubicin and methotrexate in advanced esophagogastric cancer. J Clin Oncol 15: 261- 267, 1997.
11. Ajani JA, Van Cutsem E, Moiseyenko S, et al: Docetaxel (D), cisplatin, 5-fluorouracil compare to cisplatin (C) and 5-fluorouracil (F) for chemotherapy-naïve patients with metastatic or locally recurrent, unresectable gastric carcinoma (MGC): Interim results of a randomized phase III trial (V325) (abstract 999). Proc Am Soc Clin Oncol 22:249, 2003.
12. Kang YK, Kim W, Chang HM, et al: Phase I-II study of docetaxel, capecitabine and cisplatin as first-line chemotherapy in advanced gastric cancer (abstract 1319). Proc Am Soc Clin Oncol 22:328, 2003.
13. Cocconi G, Carlini P, Gamboni A, et al: Cisplatin, epirubicin, leucovorin and 5-fluorouracil (PELF) is more active than 5-fluorouracil, doxorubicin and methotrexate (FAMTX) in advanced gastrica carcinoma. Ann Oncol 14:1258-1263, 2003.
14. Vanhoefer U, Rougier P, Wilke H, et al: Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 18:2648-2657, 2000.
15. Chollet P, Schoffski P, Weigang-Kohler K, et al: Phase II trial with S-1 in chemotherapy– naïve patients with gastric cancer. A trial performed by the EORTC Early Clinical Studies Group (ECSG). Eur J Cancer 39:1264- 1270, 2003.
16. Sasaki T: Current topics of S-1 at the 74th Japanese Gastica Cancer Congress. Gastric Cancer 6(suppl 1):9-12, 2003.
17. Koizumi W, Saigenji K, Ujiie S, et al: A pilot phase II study of capecitabine in advanced or recurrent gastric cancer. Oncology 64:232- 236, 2003.
18. Maeda S, Saikawa Y, Kubota T, et al: No cross-resistance of taxotere and taxol to conventional chemotherapeutic agents against gastric cancers as detected by MTT assay. Anticancer Res 23:3147-50, 2003.
19. Wilke HJ, Van Cutsem E: Current treatments and future perspectives in colorectal and gastric cancer. Ann Oncol 14(suppl 2):49-55, 2003.
20. Garcia-Carbonero R, Supko JG: Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clin Cancer Res 8:641-61, 2002.
21. Kohne CH, Catane R, Klein B, et al: Irinotecan is active in chemonaive patients with metastatic gastric cancer: A phase II multicentric trial. Br J Cancer 89:997-1001, 2003.
22. Sumpter K, Harper-Wynne C, Cunningham D, et al: Randomized, multicenter phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced esophagogastric cancer: Confirmation of dose escalation (abstract 1031). Proc Am Soc Clin Oncol 22:257, 2003.
23. Bajetta E, Celio L, Buzzoni R, et al: Phase II study of pemetrexed disodium (Alimta) administered with oral folic in patients with advanced gastric cancer. Ann Oncol 14:1543-1548, 2003.
24. Leichman L, Pendyala L, Leichmman CG: Definitive and neoadjuvant therapies for esophageal and gastroiesophageal junction tumors: A look back and toward the future. Semin Oncol 30(4 suppl 11):11-18, 2003.