Translational Research With Pemetrexed in Breast Cancer
Pemetrexed (Alimta) is a novel folate antimetabolite that primarilyinhibits the enzymes thymidylate synthase (TS), dihydrofolate reductase(DHFR), and glycinamide ribonucleotide formyl transferase(GARFT), all of which are involved in pyrimidine and purine synthesis.In a phase II trial of patients with T3/4, N0–2 breast cancer, expressionof thymidylate synthase (TS), dihydrofolate reductase (DHFR),glycinamide ribonucleotide formyltransferase (GARFT), p53, andc-erb-B2 (at the mRNA or protein level) was examined in tumor biopsyspecimens before and 24 hours after the first dose of pemetrexed andafter three cycles of single-agent treatment to establish correlations ofbiomarker levels and changes with clinical outcome and toxicity. Althoughfinal data are not available, initial indications are that clinicalresponse may correlate with decreased or low TS expression. The resultsobtained from clinical data are supported by laboratory results inthree cell lines (MDA-231, MCF-7, and ZR-75). These results suggestthat in vitro transcript profiling to identify which genes are importantpredictors of successful cytotoxic chemotherapy, followed by a focusedclinical trial to confirm the in vitro results, may be the best approachfor translational research.
ABSTRACT: Pemetrexed (Alimta) is a novel folate antimetabolite that primarilyinhibits the enzymes thymidylate synthase (TS), dihydrofolate reductase(DHFR), and glycinamide ribonucleotide formyl transferase(GARFT), all of which are involved in pyrimidine and purine synthesis.In a phase II trial of patients with T3/4, N0â2 breast cancer, expressionof thymidylate synthase (TS), dihydrofolate reductase (DHFR),glycinamide ribonucleotide formyltransferase (GARFT), p53, andc-erb-B2 (at the mRNA or protein level) was examined in tumor biopsyspecimens before and 24 hours after the first dose of pemetrexed andafter three cycles of single-agent treatment to establish correlations ofbiomarker levels and changes with clinical outcome and toxicity. Althoughfinal data are not available, initial indications are that clinicalresponse may correlate with decreased or low TS expression. The resultsobtained from clinical data are supported by laboratory results inthree cell lines (MDA-231, MCF-7, and ZR-75). These results suggestthat in vitro transcript profiling to identify which genes are importantpredictors of successful cytotoxic chemotherapy, followed by a focusedclinical trial to confirm the in vitro results, may be the best approachfor translational research.
During the past decade, the integrationof translational researchinto clinical trials hasbecome an important aspect of cancerresearch. The goal of pharmacogenomicanalysis is to identify individualswith specific genetic characteristicsor molecular variables thatcorrespond with clinical response orresistance. These analyses may alsoserve as a guide in developing targetedtherapies that may improve tumor responseand, ultimately, patient survival.The utility of translational researchand the impact of patient selection fortargeted therapies have been investigatedin a variety of tumors. However,breast cancer has been the tumorprobably most extensively studied todate, and several prognostic factorshave been well established. The etiologyof breast cancer is complex and itappears to involve numerous genetic,endocrine, and external factors. Mostlikely, a combination of events is requiredfor the formation of cancer includingoverexpression of oncogenesthat direct a cell to divide, inhibitionof signals to stop replication or loss of maintenance of integrity of the genomeby defunct tumor suppressorgenes, and/or DNA replication andrepair defects.[1]Available data in translational researchmay be confusing to oncologistswho need to translate this datainto therapeutic decisions. Translationalclinical trials are under way todetermine which genes are importantpredictors of cytotoxic chemotherapyresponse and resistance.Pemetrexed (Alimta) is a novelfolate antimetabolite that inhibitsthymidylate synthase (TS), dihydrofolatereductase (DHFR), and glycinamideribonucleotide formyltransferase (GARFT), all of which areinvolved in pyrimidine and purinesynthesis,[2,3] resulting in impededsynthesis of the nucleotide precursorsof DNA and RNA.
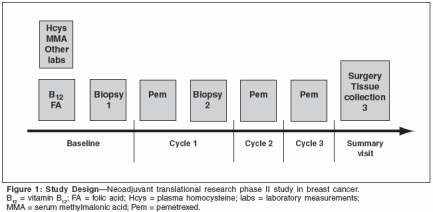
Phase II TrialDesign and Patient Characteristics
A phase II neoadjuvant trial ofpemetrexed in patients with breastcancer has been conducted. In thisstudy, biomarker expression was evaluatedin patients with T4, N0-2, M0/1 breast cancer. Biopsies were obtainedprior to the initial dose of pemetrexed,24 hours after administrationof pemetrexed, and after three cyclesof therapy. Antifolates have been associatedwith severe sporadic toxicity,and evidence suggests that vitaminsupplementation with folic acid andvitamin B12 reduces plasma homocysteinelevels, leading to a better safetyprofile with pemetrexed without adverselyaffecting efficacy.[4,5] As aresult, baseline vitamin deficiencymarkers were drawn, and vitamin supplementationwas given prior to administrationof pemetrexed. Thedesign of this study is shown in Figure1.The median age of enrolled patientswas 46 years of age (range: 31to 72 years). The majority of patients(93%) received three cycles of therapy.The overall response rate was 31%(95% confidence interval [CI] = 20%to 44%). Fifteen patients (79%) exhibitedthe first evidence of a responseafter the first cycle.
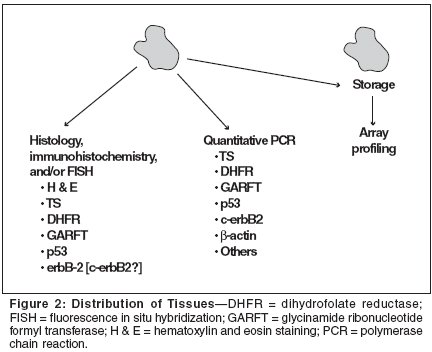
The emphasis of the biopsy tissueassessment was placed on messengerRNA (mRNA) expression of TS,DHFR, and GARFT with additionalstudies including quantitative polymerasechain reaction of p 53, andC-erb B2 as well as histology, immunohistochemistry,and/or fluorescencein situ hybridization (FISH) for TS,DHFR, GARFT, p53, and C-erb B2.For measurement of GARFT, a monoclonalantibody was specifically developedfor this study. Additionalmaterial was retained and stored forlater gene expression profiling. Frozenand paraffin-embedded specimenswere evaluated to ensure that theycontained sufficient tumor tissue forhistologic analysis. Ninety-four percentof frozen specimens and 88% ofparaffin-embedded specimens containedsufficient tumor tissue and wereevaluable for histologic analysis.(The tissue analyses are illustrated inFigure 2.)
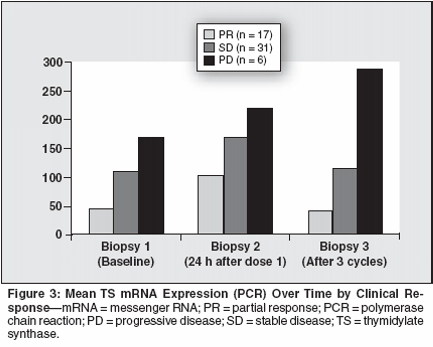
Preliminary Results
Preliminary results demonstratethat there was no real difference inHER2/neu classification between respondersand nonresponders. Analysesof p53 mutations are still ongoing.However, preliminary data indicatedthat there were no p53 mutations inresponders, and four mutations wereobserved in nonresponders. Usingimmunohistochemistry, no clear differencebetween TS responders andnonresponders was noted; however,the distribution of baseline TS as determinedby polymerase chain reaction(PCR) revealed that there was alarger distribution in nonresponderswith a trend toward higher values,and a more close clustering of low TSvalues in responding patients.When TS was determined and normalizedto beta-actin, three groups emerged-very low TS expression,intermediate TS expression, and higherTS expression. Generally, up to90% of the responding patients fellinto the very low or lower intermediateTS expression as determined bymRNA and PCR analyses. When examiningthe data over time (ie, baseline,after the first dose of pemetrexed,following three cycles), questions regardingthe ability of pemetrexed toinduce resistance and differences inTS, and whether or not pemetrexedcan modulate TS expression in patientsfollowing treatment were addressed.Preliminary analysis indicated thatin responders (n = 17 with objectivepartial responses) and patients with stabledisease (n = 31), pemetrexed doesnot induce TS expression. This alsoappears to be true among patients withstable disease; however, in resistantpatients with progressive disease (n =6), it appears that treatment with pemetrexedover time increased TS expressionby a factor of two. Figure 3illustrates differential TS expression inresponders, patients with stable disease,and patients with disease progression.These preliminary data suggest thatpemetrexed may not upregulate TS overtime in those patients who benefit fromthe therapy. The role of DHFR,GARFT, and other molecular markersis currently under evaluation.The results of this type of translationalresearch may prove to be oneof the best indicators of a patient'spotential response to cytotoxic chemotherapy;however, applying thistechnology in the clinical setting is animmense logistical challenge for allinvolved. Is it possible that modelingcan provide the necessary informationfor making clinical treatment decisions?Three breast cancer celllines-which included MDA 231, avery aggressive, estrogen receptornegative cell line; MCF7, a moderatelyaggressive cell line; and ZR-75a less malignant cell line-were examined.With respect to TS, a consistenttwofold increase in TS expressionwas noted, which corresponds to theclinical results observed.DiscussionThere are two approaches forconducting translational research,the first being clinical studies. Forclinical studies tumors accessiblefor repetitive biopsies are required,limiting the number of possible candidatesto leukemia, certain kindsof breast cancer, head and neck cancer,and melanomas with cutaneousmetastasis. These studies are difficultto perform. Accrual is slow,logistics are very complex, andthese studies are very costly.The other opportunity for performingtranslational research is to conductsome preliminary work withpatient-derived material. Using thisapproach, a large number of accessibletumors are available, because generallyevery cancer patient is operatedon, thus increasing the opportunityof tissue accrual. However, verificationin subsequent prospective clinicaltrials would be required, but invitro investigations will allow inves-tigators to decrease the number ofpotential prognostic candidate genes.This clinical approach could then bemore focused than exploring numerousparameters, many of which willultimately be eliminated. Both approachesfor translational researchrequire a centralized and validatedlaboratory.In conclusion, pemetrexed has demonstratedclinical activity in untreatedbreast cancers. Preliminary data indicatethat the clinical response may correlatewith decreased or low TSexpression. Gene expression profilingusing several hundred genes is inprogress. The challenge, however, willbe to sort out which genes are reallyimportant predictors of successful clinicaltreatment with pemetrexed. Analysesof biopsies during the treatmentwill provide information on the pemetrexed-induced modulation of genes atfunctional levels.
Disclosures:
Dr. Hanauske hasacted as a consultant for Eli Lilly.
References:
1.
Ferrigno PK: The application of basicscience to translational cancer research.Genome Biol 4:305, 2003. E-pub January30, 2003.
2.
Boyer MJ, Rivory LP, Clarke SJ:Pemetrexed: Single-agent and combinationphase I study overview. Semin Oncol 29(suppl18):18-23, 2002.
3.
Schultz RM, Patel VF, Worzalla JF, etal: Role of thymidylate synthase in the antitumoractivity of the multitargetedantifolate, LY231514. Anticancer Res19:68-73, 1999.
4.
Niyikiza C, Baker SD, Seitz DE, et al:Homocysteine and methylmalonic acid: Markersto predict and avoid toxicity frompemetrexed therapy. Mol Cancer Ther 1:545-552, 2002.
5.
Niyikiza C, Hanauske AR, Rusthoven JJ,et al: Pemetrexed safety and dosing strategy.Semin Oncol 29(6 suppl 18):24-29, 2002.