Overview of Phase I/II Pemetrexed Studies
Pemetrexed (Alimta) is an antifolate that is effective in the inhibitionof multiple enzyme targets including thymidylate synthase,dihydrofolate reductase, and glycinamide ribonucleotide formyl transferase.The compound has been evaluated in several phase I trials, bothas single agent and in combination with other cytotoxic agents. Theinitial schedule selected for further investigation in phase II trials waspemetrexed 600 mg/m2 as a 10-minute infusion on day 1 every 21 days.During the subsequent phase II development, the dose of pemetrexedwas adjusted to 500 mg/m2 due to bone marrow and gastrointestinaltoxicities. The adjusted dose of pemetrexed was well tolerated throughoutthe late-phase drug development program. Preclinical evidencesuggests that pemetrexed has additive or synergistic activity when combinedwith many other clinically important anticancer agents, includinggemcitabine (Gemzar), fluorouracil, carboplatin (Paraplatin),oxaliplatin (Eloxatin), paclitaxel, and vinorelbine (Navelbine). Doselimitingtoxicities in these studies were primarily hematologic, and therewas no evidence of cumulative hematologic toxicity. During the drugdevelopment program it was discovered that supplementation with folicacid and vitamin B12 profoundly increased the tolerability ofpemetrexed. The studies discussed in this review demonstrate thatpemetrexed is well tolerated as a single agent and will be an importantcontribution to combination chemotherapy regimens.
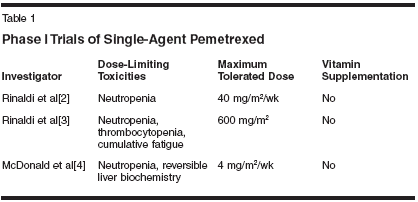
ABSTRACT: Pemetrexed (Alimta) is an antifolate that is effective in the inhibitionof multiple enzyme targets including thymidylate synthase,dihydrofolate reductase, and glycinamide ribonucleotide formyl transferase.The compound has been evaluated in several phase I trials, bothas single agent and in combination with other cytotoxic agents. Theinitial schedule selected for further investigation in phase II trials waspemetrexed 600 mg/m2 as a 10-minute infusion on day 1 every 21 days.During the subsequent phase II development, the dose of pemetrexedwas adjusted to 500 mg/m2 due to bone marrow and gastrointestinaltoxicities. The adjusted dose of pemetrexed was well tolerated throughoutthe late-phase drug development program. Preclinical evidencesuggests that pemetrexed has additive or synergistic activity when combinedwith many other clinically important anticancer agents, includinggemcitabine (Gemzar), fluorouracil, carboplatin (Paraplatin),oxaliplatin (Eloxatin), paclitaxel, and vinorelbine (Navelbine). Doselimitingtoxicities in these studies were primarily hematologic, and therewas no evidence of cumulative hematologic toxicity. During the drugdevelopment program it was discovered that supplementation with folicacid and vitamin B12 profoundly increased the tolerability ofpemetrexed. The studies discussed in this review demonstrate thatpemetrexed is well tolerated as a single agent and will be an importantcontribution to combination chemotherapy regimens.Pemetrexed (Alimta) is a novelmultitargeted antifolate antimetabolitethat inhibits, amongother enzymes, thymidylate synthase(TS), dihydrofolate reductase(DHFR), and glycinamide ribonucleotideformyl transferase (GARFT).The primary targets of pemetrexedcontrol pivotal steps in the de novosynthesis of pyrimidines and purines.The multitargeted nature of pemetrexedis confirmed by in vitro experimentsdemonstrating that both thymidineand hypoxanthine are requiredto prevent pemetrexed-induced cytotoxicity.[1]Three initial single-agent phase Istudies were conducted to explore differenttreatment schedules of pemetrexed.These schedules comprisedadministration of the compound weekly* 4 every 6 weeks, daily for 5 daysevery 21 days, and once every 21days. Pemetrexed was administeredas a 10-mg/m2 intravenous (IV) infusionover 10 minutes.Phase I Trials ofSingle-Agent PemetrexedRinaldi et al reported a single-agentphase I study with pemetrexed administeredas a 10-minute infusionevery week for 4 weeks. The treatmentwas repeated after 6 weeks in patientswith advanced solid tumors.[2] A totalof 25 patients received doses rangingfrom 10 to 40 mg/m2/wk. The doselimitingtoxicity consisted of neutropeniathat was completely reversible.Nonhematologic toxicities were mild,and no grade 3 or 4 nonhematologictoxicities were reported. The maximumtolerated dose was determinedto be 40 mg/m2/wk and the recommendedphase II dose utilizing thisadministration schedule was 30 mg/m2/wk. Two patients with advancedcolorectal cancer experienced minor responses.Based on the results of this study,Rinaldi and colleagues conducted asecond phase I trial.[3] In this study,pemetrexed was administered as IVinfusion over 10 minutes every 21days. A total of 37 patients with advancedsolid tumors received dosesranging from 50 to 700 mg/m2. Themaximum tolerated dose was foundto be 600 mg/m2 and the recommendeddose for subsequent phase II trialswas determined to be 600 mg/m2. Neutropenia,thrombocytopenia, and cumulativefatigue were dose-limitingtoxicities. Partial responses were notedin patients with advanced pancreaticcancer (n = 2) and advancedcolorectal cancer (n = 2), with minorresponses in patients with advancedcolorectal cancer (n = 6).The third single-agent phase I trialwith pemetrexed was conducted byMcDonald and colleagues.[4] In thisstudy, pemetrexed was administeredas an IV infusion over 10 minutesdaily for 5 days. The treatment wasrepeated every 21 days. Thirty-eightpatients with advanced malignanciesreceived pemetrexed with doses rangingfrom 0.2 to 5.2 mg/m2. The maximumtolerated dose was found to be 4mg/m2, with neutropenia being the leadingdose-limiting toxicity. One patientwith metastatic non-small-cell lungcancer (NSCLC), one with metastaticcolon cancer, and one with pancreaticcancer experienced minor responses.A comparison of the results fromthese three phase I studies indicated arelationship between the maximum tolerateddose treatment schedules; however,myelosuppression and epithelialtoxicities as manifested by mucositisand/or diarrhea remained as predominanttoxicities (Table 1). Additionalnonhematologic toxicities included fatigueand rash. Because of the convenientadministration schedule, theachievable dose intensity, and the extentof anecdotal antitumor activity observed,the every-21-day schedule wasselected for further development ofpemetrexed. The recommended phaseII dose of 600 mg/m2 was subsequentlyreduced to 500 mg/m2 in order to furtheroptimize the tolerability of pemetrexedand to prepare for early clinicaltrials with combination regimens.None of the single-agent phase Istudies used supplementation of patientswith folic acid and vitamin B12.The added value of vitamin supplementationto the safety of pemetrexedwas only discovered after the agenthad already entered its first registrationphase III registration trial.Pharmacokinetic parameters ofpemetrexed were evaluated in thesephase I studies and yielded the followingconclusions: at clinically useddose ranges, pemetrexed is eliminatedfrom serum, with a mean terminalelimination half-life of 2 to 3 hours.[5]A linear relationship between areaunder the concentration-time curve(AUC) and dose was noted. Within24 hours after administration of thecompound, 70% to 90% of the administereddose is recovered in theurine. Hepatic metabolism of parentcompound is minimal leading to negligibleamounts of biologically inactivemetabolites. There was noevidence for renal toxicities of pemetrexedin patients with normal creatinineclearance despite the fact thatpemetrexed is renally eliminated.Nevertheless, in order to determinewhether comedication with potentiallynephrotoxic agents may lead to renaltoxicities, a phase I trial wasrecently completed which evaluatedthe effects of combining ibuprofenwith pemetrexed in patients with advancedcancer. Preliminary pharmacokineticdata indicated thatcoadministration of these two agentsdid not affect creatinine clearance orpharmacokinetic variables of pemetrexed.[6]
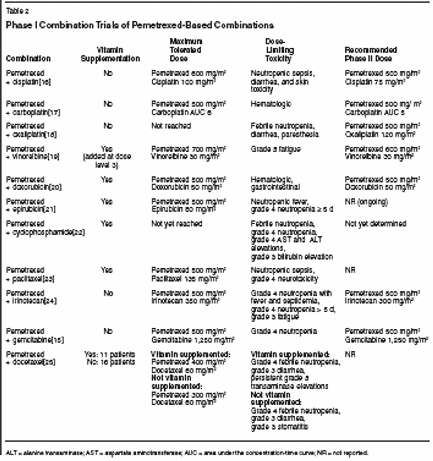
Combination RegimensWith PemetrexedPemetrexed underwent extensiveevaluation in preclinical studies todetermine if the agent could be combinedwith other clinically used cytotoxicagents, including platinums,gemcitabine (Gemzar), cyclophosphamide(Cytoxan), the taxanes, doxorubicin,vinorelbine (Navelbine), andradiation.[7] Findings of synergisticor additive antitumor effects in humantumor xenografts and cell linessuggested that these combinationswarranted further evaluation in clinicaltrials.Table 2 summarizes clinical studiesthat have been conducted with pemetrexedcombined with a variety of otherclinically relevant compounds, and detailswill be presented below. Thesetrials have demonstrated that pemetrexedis a versatile and well-tolerateddrug that can be combined at full doseswith all compounds studied, laying thebasis for a broad subsequent drug developmentprogram.Pemetrexed CombinationWith Gemcitabine
The cytotoxicity and potential underlyingmechanisms of the combinationof pemetrexed and gemcitabinehave been evaluated in several preclinicalstudies involving a variety oftumors and using simultaneous andsequential administration.[8-14] Datahave shown that the combination resultsin synergistic cytotoxicity whenadministered sequentially but antagonismwith concurrent administration.Optimal synergy was observed withthe pemetrexed → gemcitabine sequencein studies of HT29 colon carcinomaxenografts[8] and MIAPaCA-2, PANC-1, and Capan-1 pancreaticcancer cell lines.[9] In contrast,the highest level of synergy in astudy of colon adenocarcinoma celllines LoVo, WiDR, and LRWZ occurredwhen gemcitabine administrationpreceded that of pemetrexed,while the reverse sequence resulted inadditive and synergistic effects.[10]In the latter trial, an increase in TSexpression, which is associated withresistance to conventional antifolates,was noted in all cell lines.Experiments in HT29 colon cancercells evaluated the cell-cycle-modulatingeffects of pemetrexed by flowcytometry as a potential mechanismto increase gemcitabine potency.[8]A decrease in HT29 proliferation ratecorrelated with an accumulation ofcells in S phase after 12 to 24 hours ofpemetrexed exposure. The authorsconcluded that synchronization ofHT29 cells by pemetrexed was effectinga change in the nucleotidepools by inhibition of target enzymes-TS, GARFT, and DHFR-that in turn potentiated cytotoxicityof exposure to gemcitabine. Other studies have also shown S-phase cellsynchronization after pemetrexedtreatment.[9,10]
In MIA PaCA-2, PANC-1, andCapan-1 pancreatic cell lines, Giovannettiet al demonstrated that pemetrexedtreatment significantlyenhanced gene expression and activityof deoxycytidine kinase (dCK), akey enzyme involved in pyrimidinesalvage pathways and in the rate-limitingstep in gemcitabine activation.[9]Rauchwerger et al studied the role ofthe equilibrative-sensitive nucleosidetransporter (es-NT) in gemcitabinesensitivity.[11] Cellular uptake ofgemcitabine requires transport acrossthe plasma membrane by sodiumindependent(equilibrative) mechanisms(es-NT), the activity of whichis a prerequisite for tumor growth inhibitionby gemcitabine.[12] Thus,combining a nucleoside analog withagents that increase NT expression,such as TS inhibitors, would theoreticallyincrease the potential for cellkill through depleting the nucleotidepool.[13,14]In experiments carried out usingTS inhibitors (5-FU and raltitrexed[Tomudex]) with gemcitabine administeredconcurrently and sequentiallyin three human pancreatic and onehuman bladder cancer cell lines, TSinhibitor pretreatment significantlyaugmented cell kill relative to singleagentgemcitabine and significantlyincreased cell surface es-NT contentover basal levels in two of the pancreaticcancer cell lines.Results were maximal when TSinhibitor treatment preceded gemcitabineadministration.[11] Thus, potentialmechanisms of synergy withthe pemetrexed → gemcitabine sequenceinclude TS inhibition, depletionof nucleotide pools, S-phasesynchronization of cells, and activationof es-NT and dCK. Mechanismsfor additive or synergistic effects observedwith the reverse sequence areless clear as yet.Based on the demonstration of preclinicalcytotoxic synergy, a phase Itrial of pemetrexed in combinationwith gemcitabine was conducted in56 patients with advanced solid tumorswho had received at least oneprevious chemotherapy regimen.[15]Adjei et al used sequential administrationof gemcitabine followed bypemetrexed, based on their in vitroclonogenic assays demonstrating cytotoxicsynergy in cultured humancolon carcinoma cells with this sequencebut not the reverse sequence.[15] Patients in group I (n =35) received gemcitabine at 1,000 or1,250 mg/m2 IV over 30 minutes ondays 1 and 8, and pemetrexed on day1 only, 90 minutes after gemcitabine,at escalating doses ranging from 200to 600 mg/m2 given IV over 10 minutes.Courses were repeated every 3weeks. Because 57% of courses wereassociated with neutropenia that requiredreduction/omission of the day8 gemcitabine dose, group II patients(n = 21) received the pemetrexed onday 8 instead of day 1.Neutropenia was the principaldose-limiting hematologic toxicity inboth groups I and II and seemed to bedose related; no infections were notedin patients with severe neutropenia.The median neutrophil count nadirwas on day 7 in group I and day 14 ingroup II patients, indicating a relationshipbetween the nadir and pemetrexedadministration. The maximumtolerated dose for group I was determinedto be gemcitabine at 1,000 mg/m2 and pemetrexed at 500 mg/m2 dueto prolonged (> 5 days) grade 4 neutropeniain four of six patients receivingthe 1,250-mg/m2 gemcitabinedose. For group II, the maximum tolerateddose was gemcitabine 1,250mg/m2 and pemetrexed 500 mg/m2 dueto life-threatening and prolonged neutropeniaseen at the higher pemetrexeddose of 600 mg/m2.The primary nonhematologic toxicitywas elevated hepatic transaminaselevel in 71% of treatment courses,most cases of which were mild tomoderate and rapidly reversible. Othertoxicities included nausea, fatigue,and rash. Patients receiving pemetrexedon day 8 (group II) had fewerand less severe toxicities and fewerdosage interruptions than those receivingpemetrexed on day 1 (group I).Among 55 assessable patients, objectiveresponses were confirmed in 7of 34 group I and 6 of 21 group IIpatients with tumors, including colorectalcancer (n = 3), non-small-celllung cancer (n = 3), cholangiocarcinoma(n = 2), ovarian cancer (n = 2),mesothelioma (n = 1), breast cancer(n = 1), and adenocarcinoma of unknownprimary site (n = 1). Twelveof these patients had partial responsesand one was considered a mixed response,with response durations of atleast 3 months. An additional 27 patientshad stable disease, with durationsof stable disease ranging from 1to 11 cycles after the initial evaluationat cycle 2. Pharmacologic evaluations conductedin four group I patients at themaximum tolerated dose showed noalteration of pemetrexed pharmacokineticsbased on gemcitabine pretreatment,although the sample sizewas small. Recommended dose andschedule of this regimen for phase IIstudy was gemcitabine 1,250 mg/m2on days 1 and 8 with pemetrexed 500mg/m2 given 90 minutes after gemcitabineon day 8, every 21 days.[15]Phase II studies of the pemetrexed/gemcitabine combination are beingcarried out in advanced-stage non-small-cell lung, breast, and pancreaticcancer, as described elsewhere inthis supplement.Pemetrexed and Cisplatin
In a phase I study of pemetrexedand cisplatin, two administrationschedules were investigated based onthe hypothesis that because pemetrexedis primarily eliminated by renalexcretion, hydration required for cisplatinadministration may potentiallymodulate the clearance of pemetrexedand thus impact on antitumor activityor toxicity.[16] In order to investigatethis hypothesis in a clinical setting,one patient cohort (n = 40) receivedpemetrexed followed by hydration andcisplatin on day 1 of a 21-day cycle.Another cohort (n = 11) was treatedwith pemetrexed on day 1 withoutany hydration, followed by hydrationand cisplatin on day 2 of a 21-daycycle. In both cohorts, pemetrexed wasadministered as an IV infusion over10 minutes.
The maximum tolerated dose forboth schedules was pemetrexed600 mg/m2 and cisplatin 100 mg/m2,demonstrating that both compounds canbe combined at fully active clinical doses. Dose-limiting toxicities consistedmainly of myelosuppression. Ten patientsin cohort 1 experienced partialresponses, and one patient with headand neck cancer had a complete response.In the second cohort, two patientsexperienced partial responses.Most notably, five of 11 patients withpleural mesothelioma developed confirmedand independently validatedpartial responses, indicating a profoundantitumor effect of this combination inmalignant pleural mesothelioma-a diseasefor which at that time no establishedtreatment was available.Antitumor responses were also notedin other tumor types including NSCLC,colorectal cancer, melanoma, and cancerof unknown primary. The recommendeddoses for subsequent clinicalstudies were determined to be pemetrexed500 mg/m2 and cisplatin 75 mg/m2 with administration of both agentson day 1.Based on the provocative results ofthis study, pleural mesothelioma waschosen as the primary target tumor entityfor approval, and additional studies,including a single-agent phase IItrial and a phase I trial with pemetrexedand carboplatin, were initiated. A courageousstep was taken by initiating theultimately successful pivotal phase IIIregistration trial based on the results ofthe phase I combination trial of pemetrexedand cisplatin.Pemetrexed in CombinationWith Carboplatin
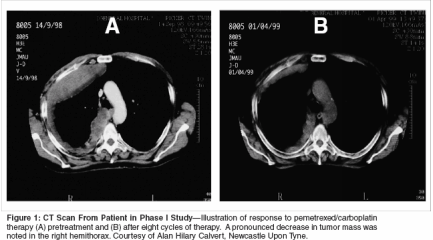
The combination of pemetrexedand carboplatin was evaluated in aphase I trial conducted by Hughesand colleagues.[17] Twenty-seven patientswith MPM received escalatingdoses of pemetrexed (400 mg/m2 to500 mg/m2) and carboplatin (AUC 4to 6). Pemetrexed was administeredas a 10-minute infusion and carboplatinwas administered as a 30-minuteinfusion, both on day 1 every 21 days.Pemetrexed at 500 mg/m2 and carboplatinat AUC 6 was the maximumtolerated dose; three of five patientsat this dose level experienced grade 4neutropenia as the dose-limiting toxicity.Nonhematologic toxicities at themaximum tolerated dose includednausea, vomiting, and stomatitis.There were no grade 4 nonhematologictoxicities reported at this doselevel. Two courses at all dose levelswere complicated by grade 3 elevationof transaminase levels. Responseto therapy was a secondary outcomeand was measured in all patients.Of the 25 patients evaluable forresponse, there were eight confirmedpartial responses, for an overall responserate of 32%. Five of the eightpatients who experienced partial responseshad stage IV disease, and fivepatients had mesothelioma of epithelialhistology. All of the patients whoreceived treatment with pemetrexedand carboplatin experienced cancerrelatedsymptoms at the start of chemotherapy.Nineteen (70%) of theoriginal 27 patients accrued experiencedrelief in cancer-related symptomswhile on study. Median overallsurvival was 451 days and mediantime to disease progression was 405days. Figure 1 shows the response ofa patient on this study. The recommendedphase II dose for this combinationwas determined to be pemetrexed500 mg/m2 and carboplatin AUC 5,which allowed for administration offull doses of both agents.Pemetrexed in CombinationWith Oxaliplatin
The combination of pemetrexedand oxaliplatin was evaluated in adose- escalating phase I clinical trialin patients with metastatic solid tumors.[18] Forty-five patients receivedpemetrexed at 300 to 500 mg/m2 followedby oxaliplatin (as a 2-hour in- fusion) at 85 to 130 mg/m2 given onday 1, every 21 days. Only 5 of 16patients experienced dose-limitingtoxicities at the highest dose level,pemetrexed 500 mg/m2 and oxaliplatin130 mg/m2; therefore, the maximumtolerated dose was not reached.Dose-limiting toxicities at this doselevel included two patients with febrileneutropenia, one patient withgrade 3 diarrhea, one patient withgrade 4 diarrhea, and one patient withgrade 3 paresthesia. There was onetoxic death at this dose level. Othertoxicities at this dose level includedmyelosuppression, diarrhea, stomatitis,and neurotoxicity. Pharmacokineticevaluation determined that therewas no evidence of pharmacokineticinteractions.Objective responses were noted in5 of 23 patients enrolled in dose levels5 and 6: one patient each withbreast cancer, rectal cancer, gastriccancer, cholangiocarcinoma, and carcinomaof unknown primary. Durationof responses ranged from 10 to14 weeks. Four of these five respondershad received at least one priorcourse of chemotherapy, and somehad received fluorouracil (5-FU) therapy.This study demonstrated thatpemetrexed can be safely combinedwith oxaliplatin at clinically activedoses of both agents. The recommendeddose of these agents when used incombination was pemetrexed at 500mg/m2 and oxaliplatin at 120 mg/m2.Pemetrexed in CombinationWith Vinorelbine
A dose-escalating phase I studywas conducted in patients with advancedor metastatic cancer.[19] Vinorelbinewas given on days 1 and 8,with pemetrexed administered on day1 of a 21-day cycle. Vitamin supplementationwas initiated at dose level3. The majority of patients had receivedprior chemotherapy and allpatients had a performance status of0 or 1. Partial responses were notedin three patients. The maximum tolerateddose was pemetrexed 700 mg/m2 and vinorelbine 30 mg/m2, wherethe dose-limiting toxicity-grade 3fatigue-was reported in two patients.One patient died of cardiac arrest thatwas not considered to be treatmentrelated. The recommended phase IIdoses of this combination regimenwere pemetrexed 600 mg/m2 on day1 and vinorelbine 30 mg/m2 on days1 and 8.[19]Phase I Studies of PemetrexedCombination Regimens inBreast CancerIn a phase I study, Hughes and colleagues[20] combined pemetrexed withdoxorubicin in 26 patients with advancedcancer. The dose of doxorubicinranged from 40 to 60 mg/m2 andpemetrexed was administered at dosesranging from 400 to 500 mg/m2. Treatmentcycles were repeated every 21days. The maximum tolerated dose wasreached at a dosage of pemetrexed 500mg/m2 combined with doxorubicin 50mg/m2. Both patients enrolled at thatdose level experienced dose-limitingtoxicities-one hematologic and onegastrointestinal. The doses recommendedfor subsequent phase II trials werepemetrexed 500 mg/m2 and doxorubicin50 mg/m2.No objective responses were noted;however, seven patients achievedstable disease after six courses of therapy.The authors recommended, basedon the documented activity of bothpemetrexed and doxorubicin, that thiscombination be studied in patientswith breast cancer.Paridaens and coworkers[21] reporteda phase I combination trial ofpemetrexed and epirubicin (Ellence)in patients with locally advanced ormetastatic breast cancer (n = 22) usingpemetrexed 400 to 500 mg/m2and epirubicin 60 to 80 mg/m2. (Thestudy patients, including 15 chemonaivepatients had not received priorchemotherapy, and completed a medianof five cycles.) The maximumtolerated dose was reached at a dosageof pemetrexed 500 mg/m2 andepirubicin 80 mg/m2. Dose-limitingtoxicities consisted of neutropeniagrade 4 and febrile neutropenia. At the time these data were reported, sevenpatients had a partial response, twohad an unconfirmed partial response,and five had stable disease with maturefollow-up. Although the maximum tolerateddose was reached at dose level 3above, accrual at pemetrexed 600 mg/m2 and epirubicin 75 mg/m2 continuedto determine the best recommendedphase II regimen. A final analysis withmature data is pending.Pemetrexed in CombinationWith Cyclophosphamide
A tumor-type-restricted phase Istudy of pemetrexed and cyclophosphamidein patients with metastaticbreast cancer was based on promisingin vitro and preclinical in vivo observations.[22] Pemetrexed was administeredover 10 minutes on day 1 of a21-day cycle as an IV infusion followedby cyclophosphamide administeredover 30 minutes as an IVinfusion approximately 20 minutesafter the start of the pemetrexed infusion.Dexamethasone (4 mg twicedaily, days 0, 1, 2) was administeredprophylactically to prevent rash. Folicacid and vitamin B12 were supplementedin order to minimizepemetrexed-induced hematologic andnonhematologic toxicities.Three to six patients were treatedat each dose level. At an interim analysis,38 patients had been entered intothe trial. The median age was 55 years(range: 32-81 years) and their performancestatus was 0 (n = 26, 68%), 1(n = 10, 26%), or 2 (n = 2, 5%). Priorchemotherapy was administered asneoadjuvant (n = 7), adjuvant (n =20), first-line metastatic (n = 4), second-line metastatic (n = 12), or thirdlinemetastatic (n = 11) therapy, withsome of the patients having receivedchemotherapy in more than one treatmentsetting.A total of 231 cycles of chemotherapywere administered with a medianof four cycles (range: 1-23cycles) per patient. At the interim evaluation,the dose level reached was1,100 mg/m2 of pemetrexed in combinationwith cyclophosphamide 600mg/m2. The latter had been escalatedup to 800 mg/m2 in combination withpemetrexed 600 mg/m2. Dose-limitingtoxicities occurred at various dose levels and included febrile neutropenia,grade 4 neutropenia, grade 4 ASTelevation, grade 4 ALT elevation, andgrade 3 bilirubin elevation. The maximumtolerated dose was not reachedand preliminary pharmacokineticevaluations indicated no modulationof pemetrexed disposition by coadministrationof cyclophosphamide.Additionally studies have been completedor ongoing with pemetrexed incombination with paclitaxel, irinotecan(Camptosar), and docetaxel (Taxotere)in phase I studies.[23-25]Pemetrexed in Combination WithRadiotherapy and Carboplatin
Based on the in vitro observationthat pemetrexed is a very potent radiationsensitizer, the combination ofpemetrexed and concurrent radiotherapywas investigated in a phase Istudy conducted by Vokes and colleagues.[26,27] Pemetrexed was administeredon day 1, every 21 days for twocycles with concurrent radiotherapy.Radiation was delivered at 2 Gy daily5 days a week for a total dose of 40 to66 Gy. Eighteen patients with NSCLC(n = 15) and esophageal carcinoma(n = 3) were enrolled. Patients couldhave received prior chemotherapy tobe eligible for the study. The protocolwas amended to include the administrationof carboplatin at AUC 4, followingpemetrexed administration.Preliminary data by Mauceri et alindicate that single-agent pemetrexedcan be combined with radiation andthat subsequent development of thiscompound also in a radiochemotherapysetting is warranted.[26] One patientexperienced a dose-limitingtoxicity consisting of grade 3 neutropeniawith infection was reported atthe pemetrexed 500-mg/m2 dose level.Grade 3 or 4 hematologic toxicitiesconsisted of grade 3 neutropeniain three patients and grade 3 hypokalemiain one; nonhematologic toxicitiesconsisted of grade 3 dysphagia in onepatient. Among 16 evaluable patients,five had a partial response, five additionalpatients had stable disease, andsix patients showed a progression oftheir disease. The authors concludedthat pemetrexed can be safely combinedwith radiation therapy at doses ofup to 600 mg/m2 with no increase of infieldtoxicities.Because platinum-containing doubletscombined with radiation therapyrepresent standard regimens in thefirst-line treatment of NSCLC, the protocolof this trial has been amended toinclude carboplatin-based regimens.The trial will subsequently enroll chemotherapy-naive patients with advancedNSCLC who will receivepemetrexed 500 mg/m2 every 21 daysfor two cycles, carboplatin at AUC 4mg/mL/min on days 1 and 22 followingpemetrexed, and radiation therapyat 2 Gy five times per week for atotal dose up to 40 to 66 Gy.Vitamin Supplementation
After it became evident that supplementationwith folic acid and vitaminB12 led to improved tolerabilityof pemetrexed, questions regardingthe optimal vitamin dose and increasingthe dose of pemetrexed in light ofthe improved safety profile arose. Inresponse to this, Hammond and colleaguesdesigned a phase I study inpatients with advanced cancer.[28]The primary objective was to determinethe maximum tolerated dose andsecondary objectives were to evaluatetoxicity and safety and antitumoractivity, to perform a pharmacokineticanalysis, and to determine recommendedphase II doses.Patients were randomly assignedto receive escalating doses of pemetrexedwith "standard-dose" vitamins(folic acid 350 to 1,000 μg orally perday) or "high-dose" folic acid (HDFA,5 mg orally per day starting 2 daysbefore administration of pemetrexedand lasting until day 3). Patients receivingHDFA were further dividedinto two groups based on the extentof their previous therapy. "Heavily"pretreated patients were defined ashaving received > 2 courses of mitomycin,> 6 courses of an alkylatingagent, > 4 courses of carboplatin, orprevious radiotherapy. Two of sixheavily pretreated patients experienceddose-limiting toxicities at thepemetrexed 925-mg/m2 dose level(grade 3 transaminase elevation, grade 4 febrile neutropenia). In these patients,the maximum tolerated dose ofpemetrexed with standard-dose vitaminsand HDFA was 800 mg/m2. Theavailable data suggest that standarddosevitamins or HDFA allow furtherdose escalation of pemetrexed; however,future studies will be needed todemonstrate whether this potential fordose increase will also result in anactual increase in therapeutic benefit,response, and survival, or not.[28]Selected Phase II Studies
Currently, five single agent phaseII studies of pemetrexed have beenperformed in patients with advancedbreast cancer.[29-33] Antitumor activitywas documented both in untreatedand heavily (> 2 prior regimensfor metastatic disease) pretreated patientsIn a study conducted by Milesand colleagues,[29] five chemotherapy-naive patients were treated withpemetrexed as first-line therapy andan additional 16 patients had beenpretreated with adjuvant chemotherapyand received pemetrexed as firstlinetherapy for metastatic disease. Theobjective response rate was 28% witha median response duration of 8.0months. However, not surprisingly, asubsequent trial demonstrated thatheavily pretreated patients who hadpreviously received anthracyclinesand taxanes as well as capecitabineshowed a lower response rate of 10%and a median response duration of5.9 months.[30]Phase II studies of pemetrexedbasedcombinations are either completedor in progress, and includecombinations with doxorubicin, epirubicin,paclitaxel, docetaxel, vinorelbine,oxaliplatin, carboplatin,gemcitabine, and cyclophosphamide.Other articles in this supplement coverthese data.ConclusionPemetrexed is a novel antifolateantimetabolite with marked singleagentand combination therapy activityin a variety of cancers, includingthoracic and breast cancers. The primarytoxicities seen in these studieswere bone marrow suppression, mucositis,diarrhea, and skin reaction.Supplementation with folic acid andvitamin B12 profoundly reduced thefrequency of grade 3 and 4 toxicitiesand further improved the tolerabilityof this agent.Pemetrexed can be safely combinedwith a large variety of clinicallyrelevant antitumor agents and thuslends itself as an optimal partner forcombination regimens. Dose-limitingtoxicities of pemetrexed in these combinationsremain myelosuppression,transaminase elevations, and epithelialside effects as demonstrated byeither mucositis and/or diarrhea.While there is no evidence for anyorgan-specific cumulative toxicities ofpemetrexed, longer-lasting treatmentswith this agent may be accompaniedby fatigue. However, this rarelyconstitutes a reason to discontinuetherapy.
Disclosures:
Dr. Hanauske hasacted as a consultant for Eli Lilly.
References:
1.
Schultz RM, Patel VF, Worzalla JF, et al:Role of thymidylate synthase in the antitumoractivity of the multitargeted antifolate,LY231514. Anticancer Res 19:437-443, 1999.
2.
Rinaldi DA, Burris HA, Dorr FA, et al: Initialphase I evaluation of the novel thymidinesynthase inhibitor, LY231514, using the modifiedcontinual reassessment method for doseescalation. J Clin Oncol 13:2842-2850, 1995.
3.
Rinaldi DA, Kuhn JG, Burris HA, et al: Aphase I evaluation of multitargeted antifolate(MTA, LY231514), administered every 21days, utilizing the modified continual reassessmentmethod for dose escalation. CancerChemother Pharmacol 44:372-380, 1999.
4.
McDonald AC, Vasey PA, Adams L, et al:A phase I and pharmacokinetic study ofLY231514, the multitargeted antifolate. ClinCancer Res 4:605-610, 1998.
5.
Sharma A, Johnson RD, Woodworth JM:Comparative human pharmacokinetics of MTAin three phase I studies (abstract 900). Proc AmSoc Clin Oncol 1998;17, 1998.
6.
Mays TA, Goetz A, Rowinsky EK, et al:A phase I trial evaluating the effects ofibuprofen and renal function on pemetrexedadministered every 3 weeks in advanced cancerpatients (abstract 2114). Proc Am Soc ClinOncol 21:75b, 2002.
7.
Bischof M, Weber KJ, Latz D, et al. Combinedeffects of MTA (multi-targeted antifolate)and ionizing radiation in vitro. J Cancer ResClin Oncol 126(suppl):R65, 2000.
8.
Tonkinson JL, Worzalla JF, Teng CH, etal: Cell cycle modulation by a multitargetedantifolate, LY231514, increases the cytotoxicityand antitumor activity of gemcitabine inHT29 colon carcinoma. Cancer Res 59:3671-3676, 1999.
9.
Giovannetti E, Mey V, Danesi R, et al:Synergistic cytotoxicity and pharmacogeneticsof gemcitabine and pemetrexed combinationin pancreatic cancer cell lines. Clin CancerRes 10:2936-2943, 2004.
10.
Tesei A, Ricotti L, DePaola F, et al: Invitro schedule-dependent interactions betweenthe multitargeted antifolate LY231514 andgemcitabine in human colon adenocarcinomacell lines. Clin Cancer Res 8:233-239, 2002.
11.
Rauchwerger DR, Firby PS, Hedley DW,et al: Equilibrative-sensitive mucleoside transporterand its role in gemcitabine sensitivity.Cancer Res 60:6075-6079, 2000.
12.
Mackey JR, Mani RS, Selner M, et al:Functional nucleoside transporters are requiredfor gemcitabine influx and manifestation oftoxicity in cancer cell lines. Cancer Res58:4349-4357, 1998.
13.
Pressacco J, Mitrovski B, Erlichman C,et al: Effects of thymidylate synthase inhibitionon thymidine kinase activity and nucleosidetransporter expression. Cancer Res55:1505-1508, 1995.
14.
Cass CE. Nucleoside transport, InGeorgopapadakou NH (ed): Antimicrobial andAnticancer Chemotherapy, pp 403-451. NewYork, Marcel Dekker Inc, 1995.
15.
Adjei AA, Erlichman C, Sloan JA, et al:Phase I and pharmacologic study of sequencesof gemcitabine and the multitargeted antifolateagent in patients with advanced solid tumors.J Clin Oncol 18:1748-1757, 2000.
16.
Thödtmann R, Depenbrock H, DumezH, et al: Clinical and pharmacokinetic phase Istudy of multitargeted antifolate (LY231514)in combination with cisplatin. J Clin Oncol17:3009-3016, 1999.
17.
Hughes A, Calvert P, Azzabi A, et al:Phase I clinical and pharmacokinetic study ofpemetrexed and carboplatin in patients withmalignant pleural mesothelioma. J Clin Oncol20:3533-3544, 2002.
18.
Missett, JL, Gamelin E, Campone M, etal: Phase I and pharmacokinetic study of themultitargeted antifolate pemetrexed in combinationwith oxaliplatin in patients with advancedsolid tumors. Ann Oncol 15:1123-1129,2004.
19.
Boyer M, Clarke S, Millward M, et al:Phase I trial of pemetrexed and vinorelbine inpatients with advanced or metastatic cancer(abstract). Ann Oncol 13:24, 2002.
20.
Hughes AN, Lind M, Azzabi A, et al: Aphase I dose escalation study of doxorubicinin combination with pemetrexed (ALIMTA,multitargeted antifolate) (abstract 438). BreastCancer Res Treat 69(3):286, 2001.
21.
Paridaens R, Dirix L, Mellaerts N, et al:Phase I study of pemetrexed (ALIMTA) andepirubicin in patients with locally advanced ormetastatic breast cancer (abstract 290). BreastCancer Res Treat 79(2):280, 2003.
22.
Dittrich C, Petruzelka L, Vodvarka P, etal: Pemetrexed and cyclophosphamide in patientswith locally advanced or metastatic breastcancer: A phase I study (abstract 159). ProcAm Soc Clin Oncol 22:40, 2003.
23.
Awada A, Clark R, Dumez H: Phase Itrial of pemetrexed plus paclitaxel administeredevery 21 days in patients with advanced solidtumors (abstract 2053). Proc Am Soc ClinOncol 23:140, 2004.
24.
Kroening H, Hochster H, Grothey A, etal: Pemetrexed and irinotecan as second-linetherapy for locally advanced or metastaticcolorectal cancer: A phase I dose escalationstudy (abstract 1459). Proc Am Soc Clin Oncol22:363, 2003.
25.
Mackay H, Carmichael J, Roberts J, etal: Phase I study of pemetrexed in combinationwith docetaxel in patients with advancedsolid malignancies (abstract 2120). Proc AmSoc Clin Oncol 21:77b, 2002.
26.
Mauceri HJ, Seetharam S, Salloum RM:Treatment of head and neck and esophagealxenografts employing Alimta and concurrentionizing radiation. Int J Oncol 19:833-835,2001.
27.
Vokes E, Szeto L, Mauer A, et al: A phaseI trial of pemetrexed + chest radiotherapy inpatients with advanced or metastatic non-smallcelllung cancer or esophageal cancer (abstractO-308). Lung Cancer 41(suppl 2):S89-90,2003.
28.
Hammond LA, Forero L, Beeram M, etal: Phase I study of pemetrexed (LY231514)with vitamin supplementation in patients withlocally advanced or metastatic cancer (abstract532). Proc Am Soc Clin Oncol 22:133, 2003.
29.
Miles DW, Smith IE, Coleman RE, etal: A phase II study of pemetrexed disodium(LY231514) in patients with locally recurrentor metastatic breast cancer. Eur J Cancer37:1366-1371, 2001.
30.
Mennel RG, O’Shaughnessy J, Blum JL,et al: Pemetrexed disodium (ALIMTA,â¢LY231514, MTA) in advanced breast cancer(ABC) patients (pts) with prior anthracyclineor anthracenedione, taxane and capecitabinetreatment: A phase II study (abstract 194). ProcAm Soc Clin Oncol 20:49a, 2001.
31.
Gomez HL, Hanauske A-R, SantillanaS, et al: A phase II trial of pemetrexed in previouslyuntreated breast cancer (abstract 225).Proc Am Soc Clin Oncol 21:57a, 2002.
32.
Llombart-Cussac A, Theodoulou M,Rowland K, et al: A phase II trial of pemetrexeddisodium (ALIMTAâ¢, LY231514, MTA) inmetastatic breast cancer (MBC) patients whohave failed anthracyclines (A) and taxanes (T)(salvage chemotherapy) (abstract 526). BreastCancer Res Treat 64(1):122, 2000.
33.
Martin M, Spielmann M, Namer M, etal: Phase II study of pemetrexed in breast cancerpatients pretreated with anthracyclines. AnnOncol 14:1246-1252, 2003.